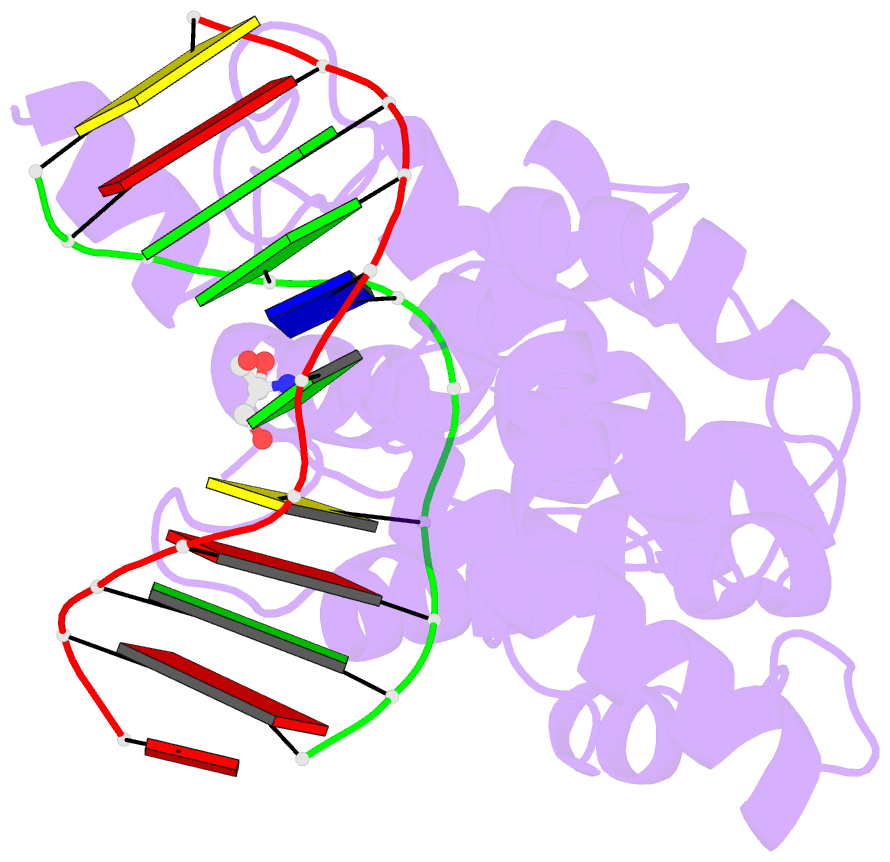
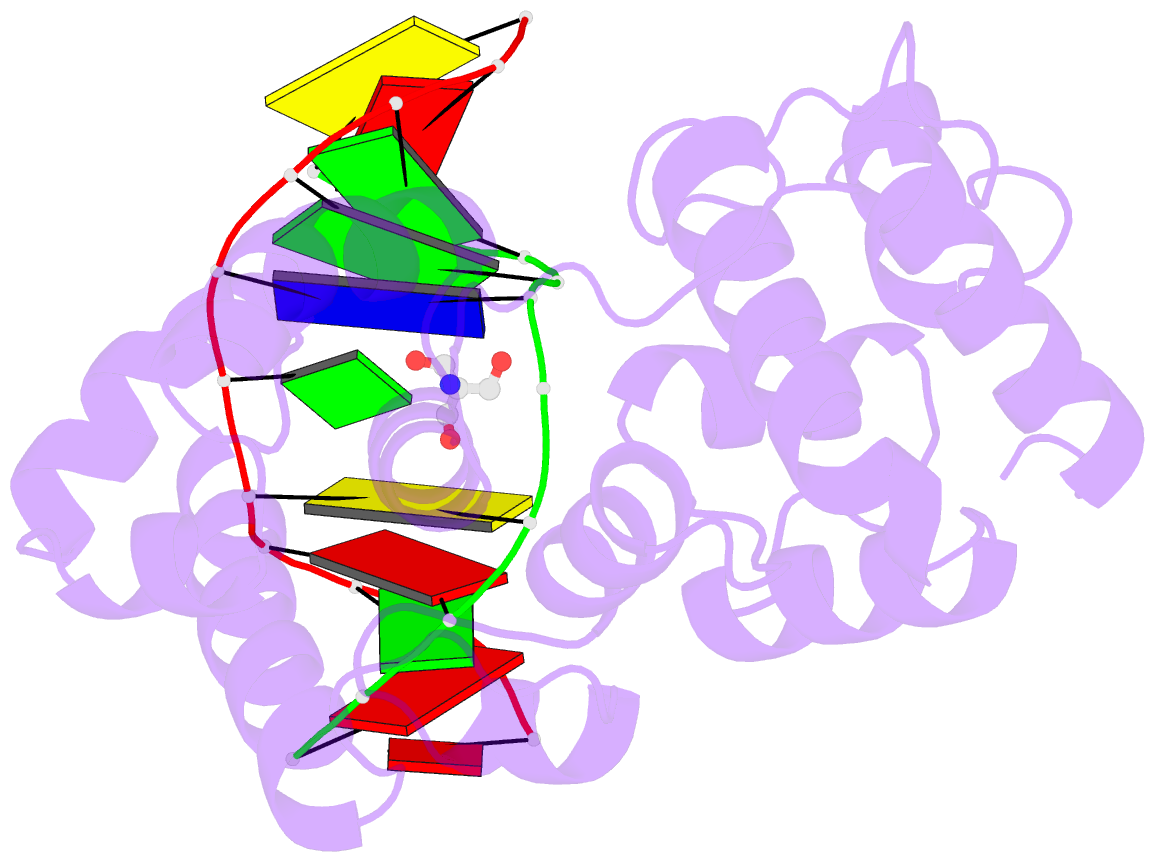
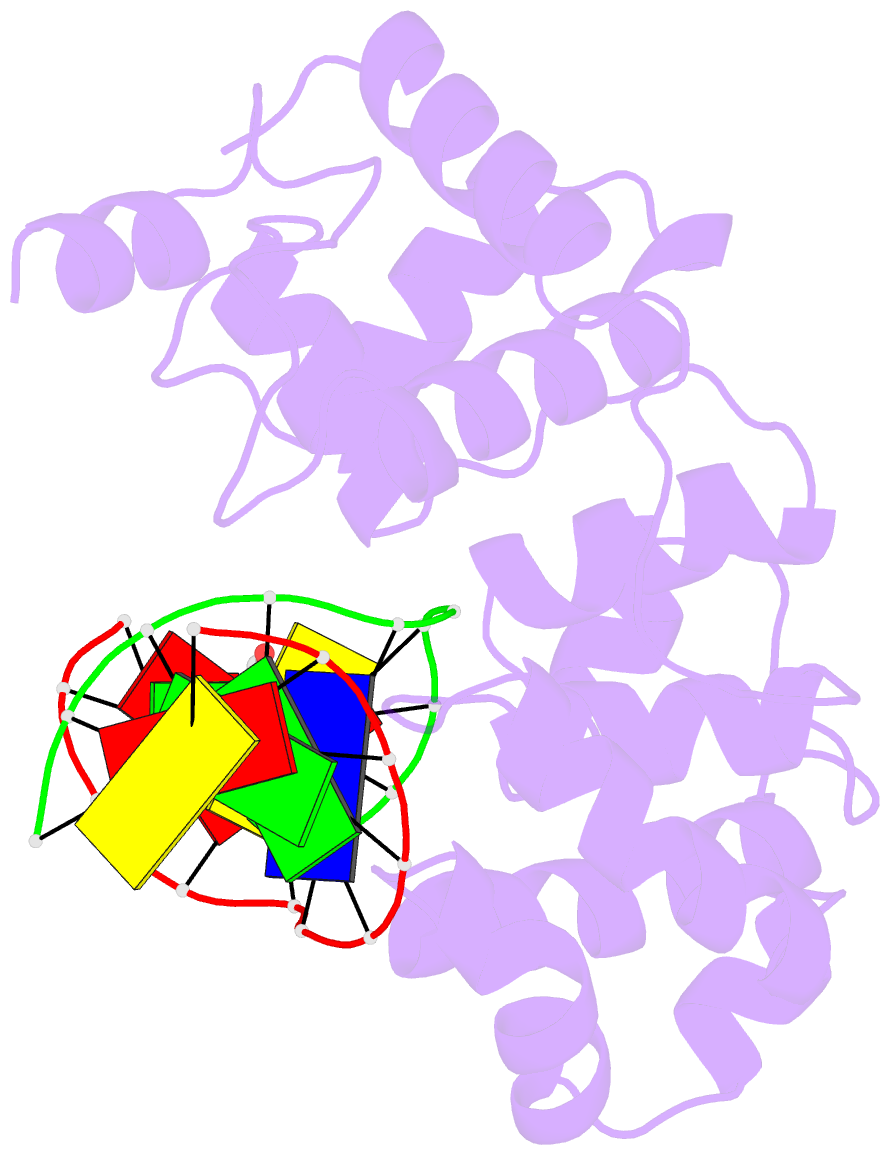
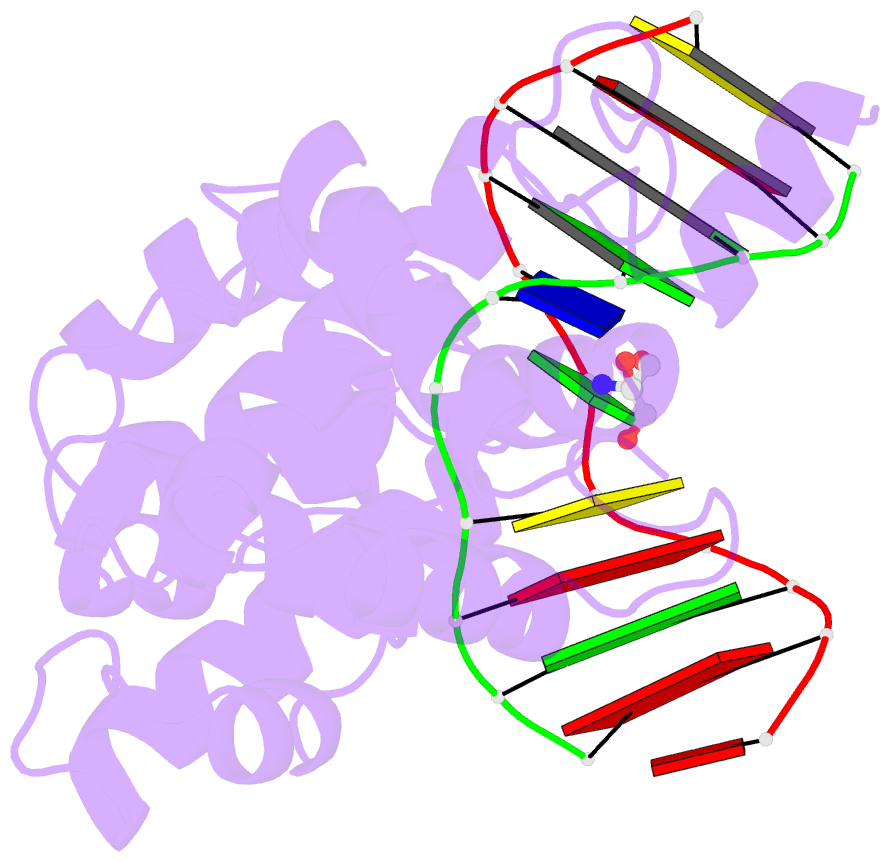
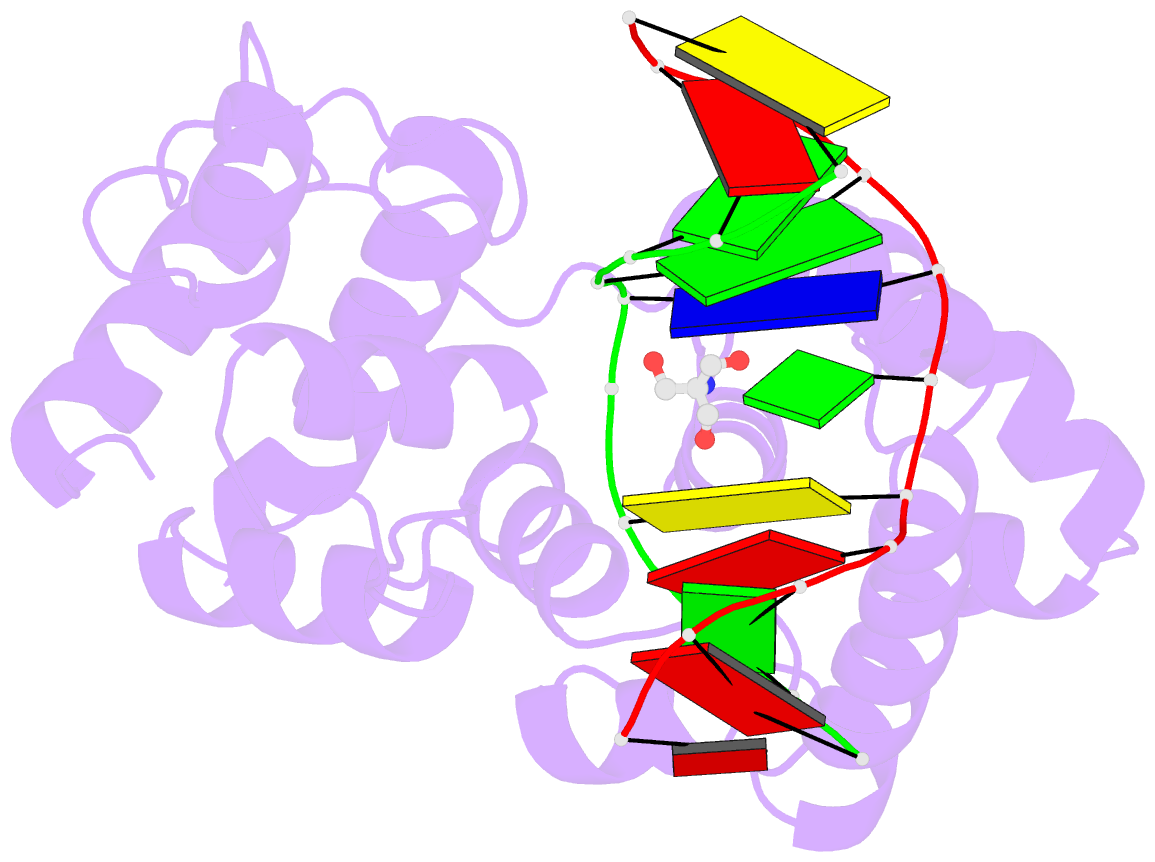
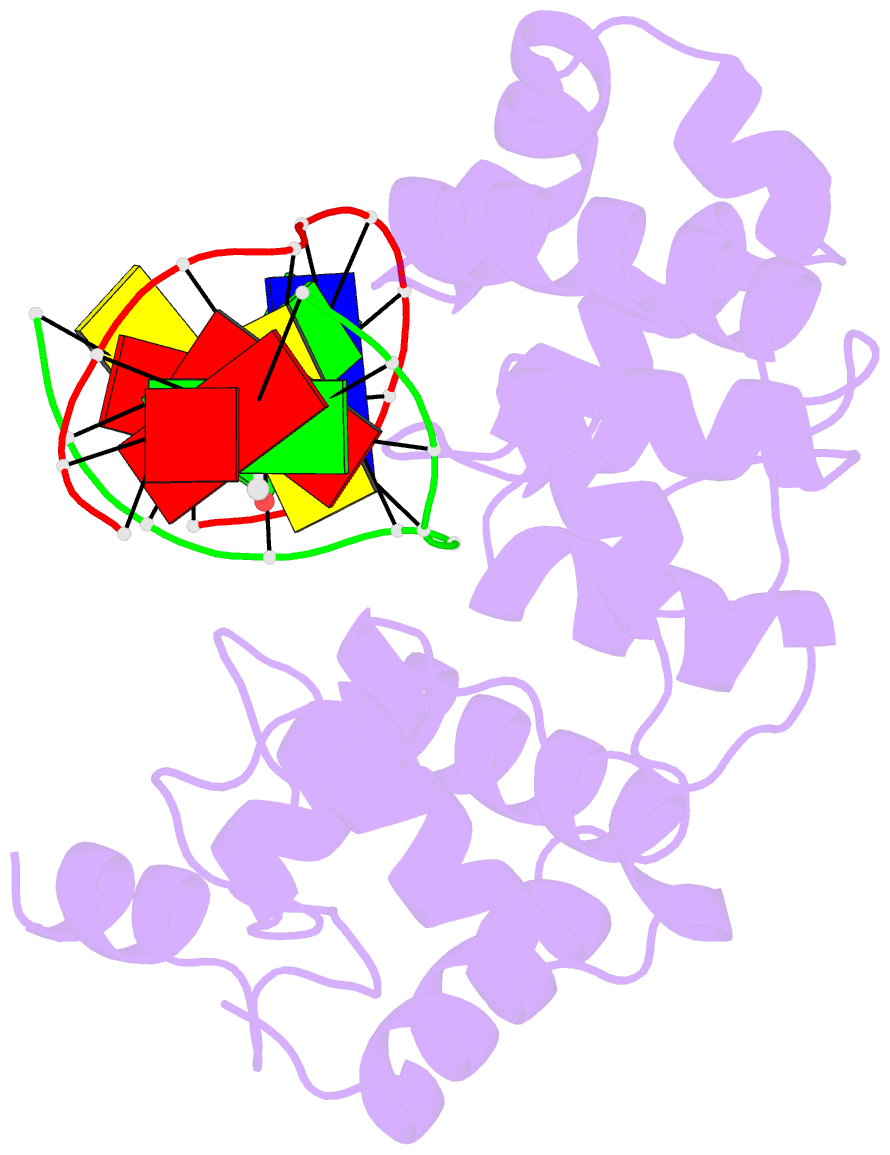
Summary information and primary citation
- PDB-id
- 1orn; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.7 Å)
- Summary
- Structure of a trapped endonuclease iii-DNA covalent intermediate: estranged-guanine complex
- Reference
- Fromme JC, Verdine GL (2003): "Structure of a Trapped Endonuclease III-DNA Covalent Intermediate." Embo J., 22, 3461-3471. doi: 10.1093/emboj/cdg311.
- Abstract
- Nearly all cells express proteins that confer resistance to the mutagenic effects of oxidative DNA damage. The primary defense against the toxicity of oxidative nucleobase lesions in DNA is the base-excision repair (BER) pathway. Endonuclease III (EndoIII) is a [4Fe-4S] cluster-containing DNA glycosylase with repair activity specific for oxidized pyrimidine lesions in duplex DNA. We have determined the crystal structure of a trapped intermediate that represents EndoIII frozen in the act of repairing DNA. The structure of the protein-DNA complex provides insight into the ability of EndoIII to recognize and repair a diverse array of oxidatively damaged bases. This structure also suggests a rationale for the frequent occurrence in certain human cancers of a specific mutation in the related DNA repair protein MYH.