Summary information and primary citation
- PDB-id
- 1pnr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.7 Å)
- Summary
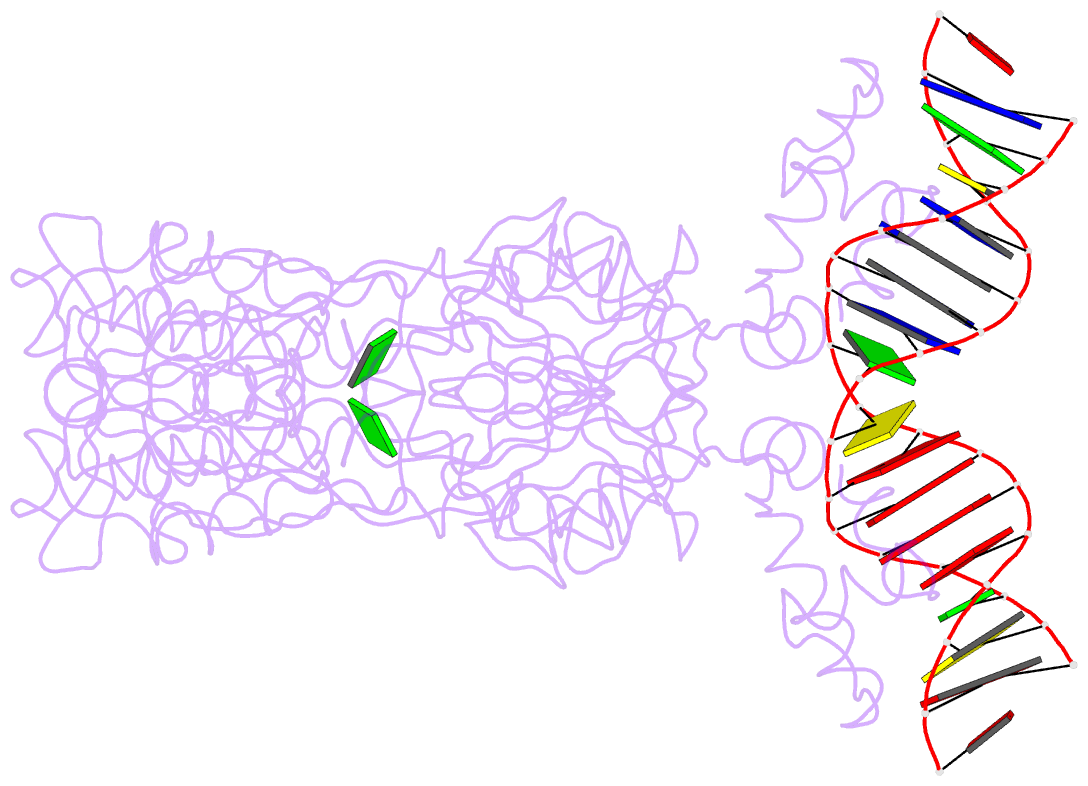
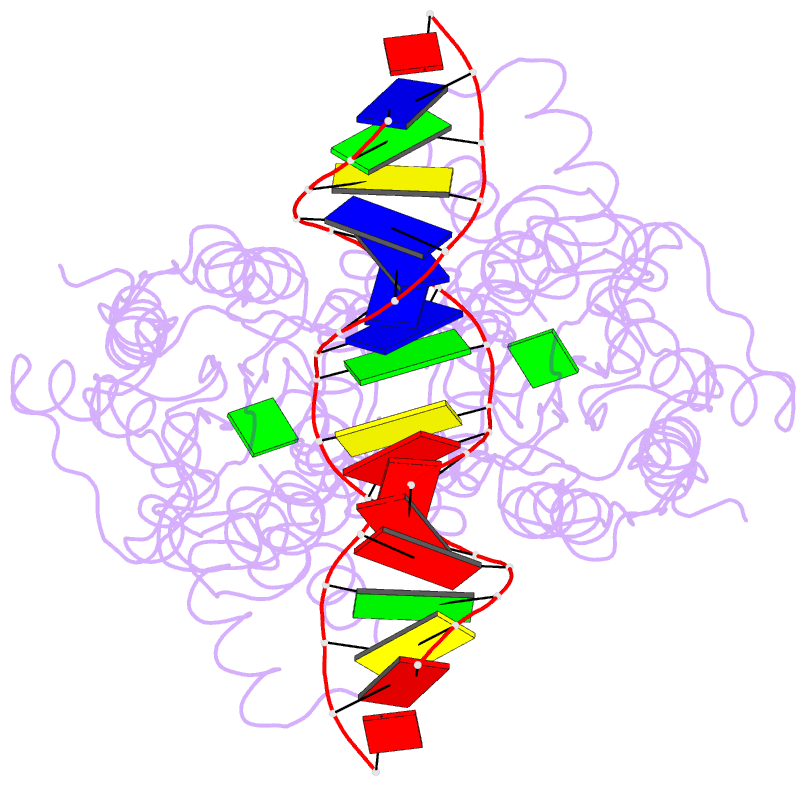
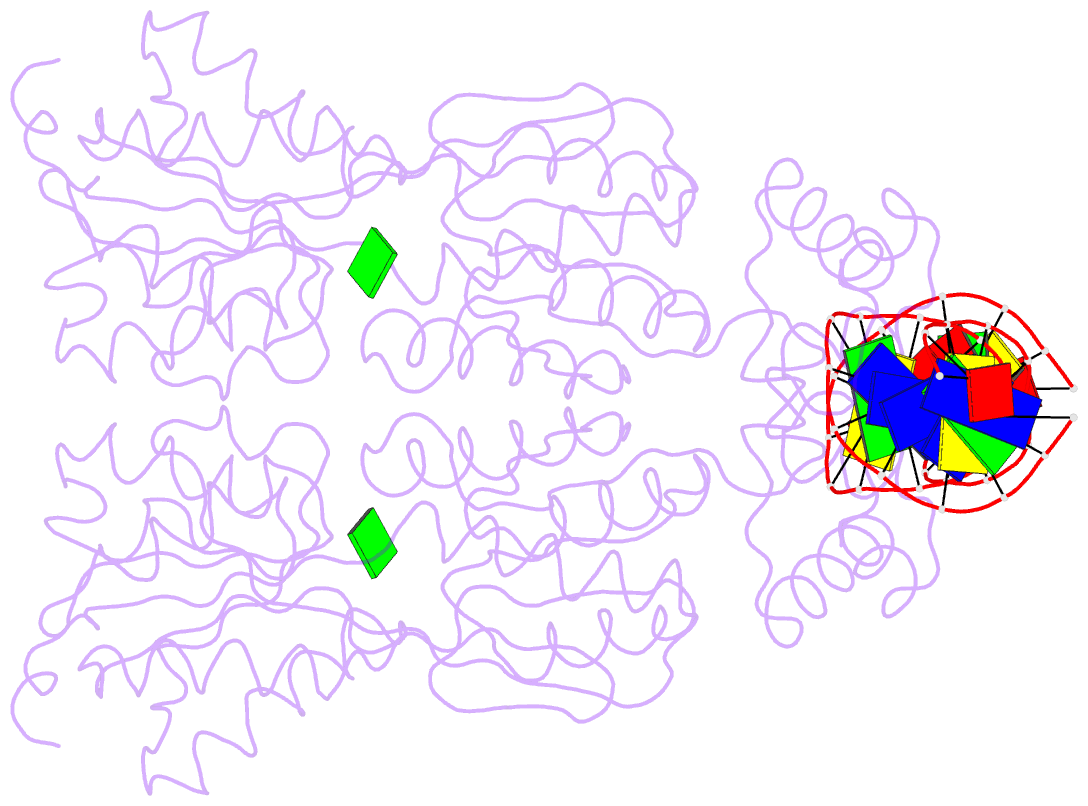
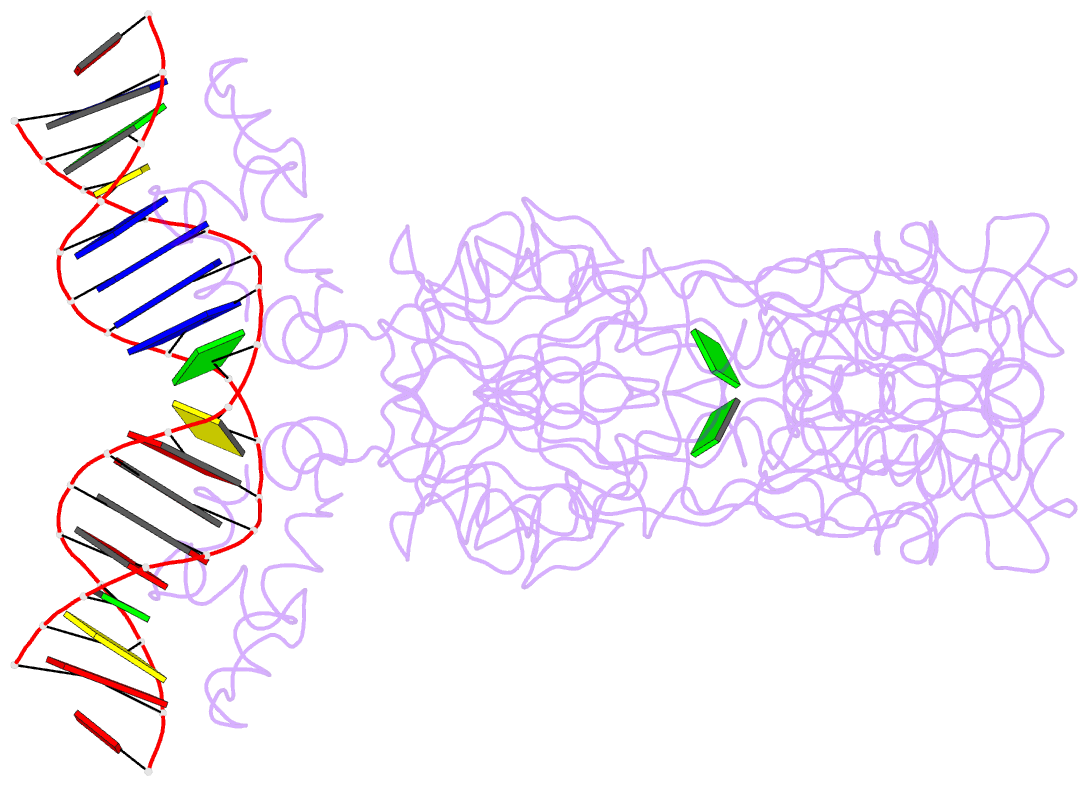
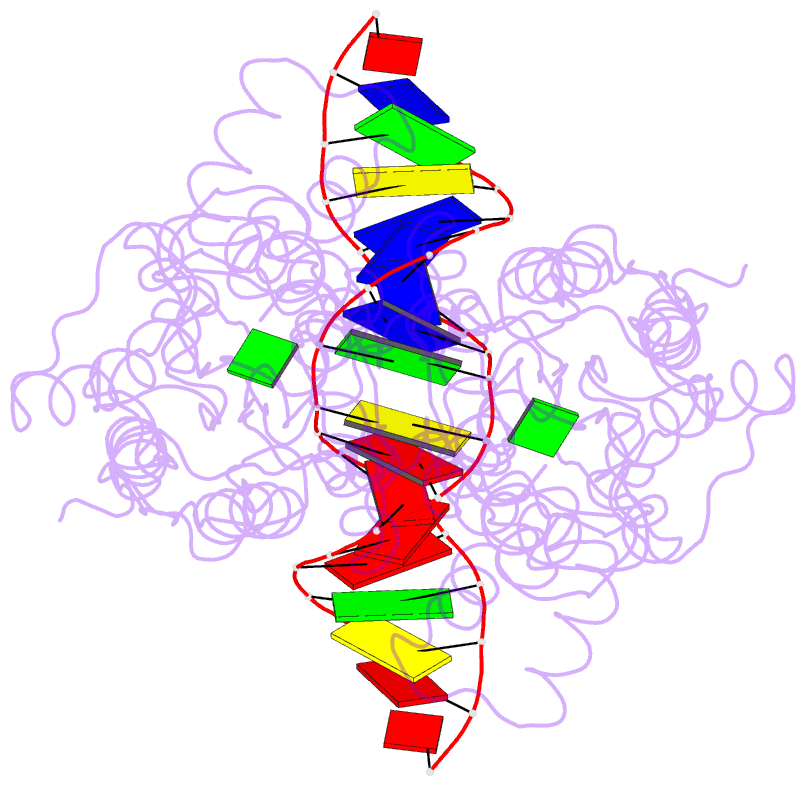
- Purine repressor-hypoxanthine-purf-operator complex
- Reference
- Schumacher MA, Choi KY, Zalkin H, Brennan RG (1994): "Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by alpha helices." Science, 266, 763-770.
- Abstract
- The three-dimensional structure of a ternary complex of the purine repressor, PurR, bound to both its corepressor, hypoxanthine, and the 16-base pair purF operator site has been solved at 2.7 A resolution by x-ray crystallography. The bipartite structure of PurR consists of an amino-terminal DNA-binding domain and a larger carboxyl-terminal corepressor binding and dimerization domain that is similar to that of the bacterial periplasmic binding proteins. The DNA-binding domain contains a helix-turn-helix motif that makes base-specific contacts in the major groove of the DNA. Base contacts are also made by residues of symmetry-related alpha helices, the "hinge" helices, which bind deeply in the minor groove. Critical to hinge helix-minor groove binding is the intercalation of the side chains of Leu54 and its symmetry-related mate, Leu54', into the central CpG-base pair step. These residues thereby act as "leucine levers" to pry open the minor groove and kink the purF operator by 45 degrees.