Summary information and primary citation
- PDB-id
- 1r0o; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.24 Å)
- Summary
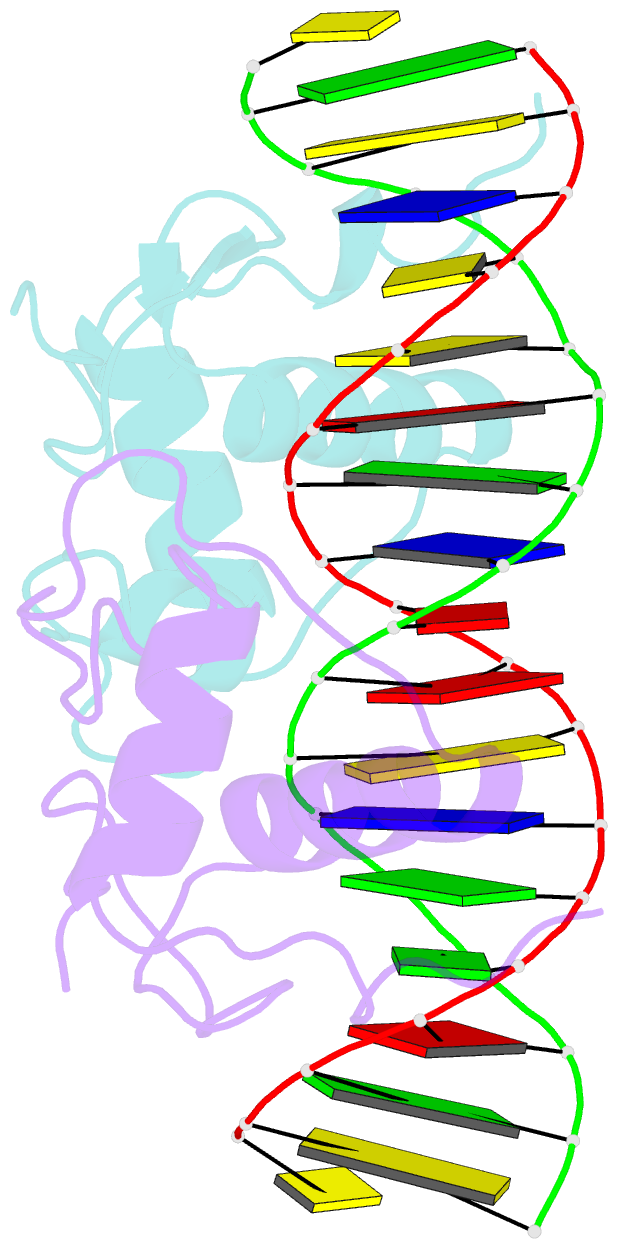
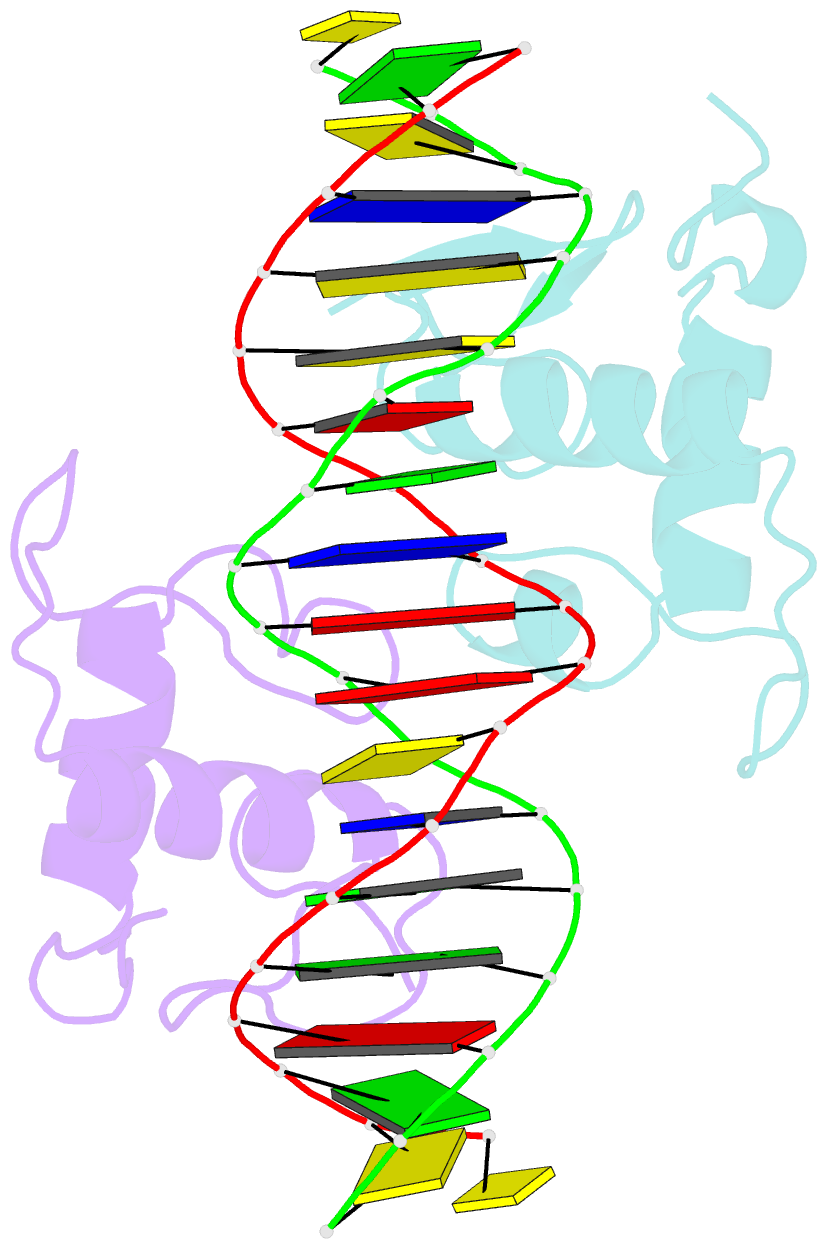
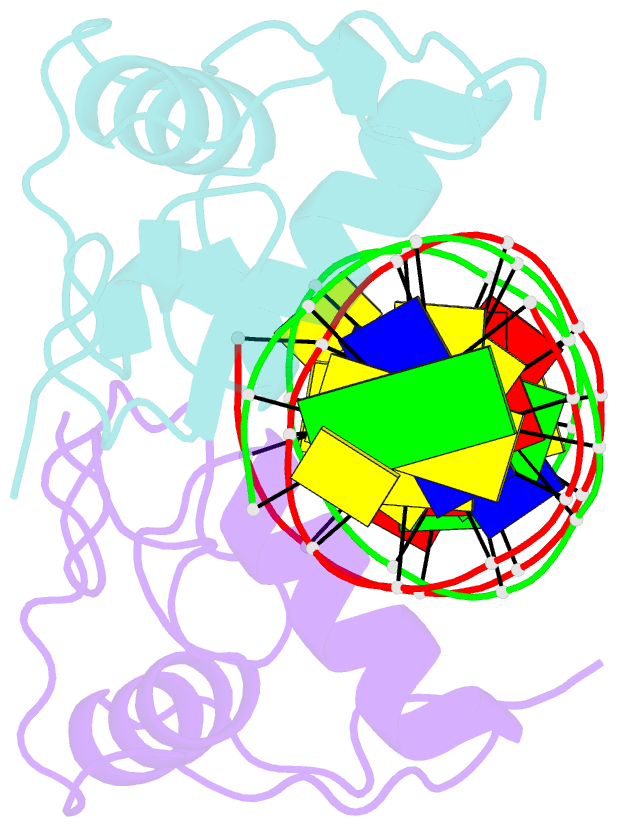
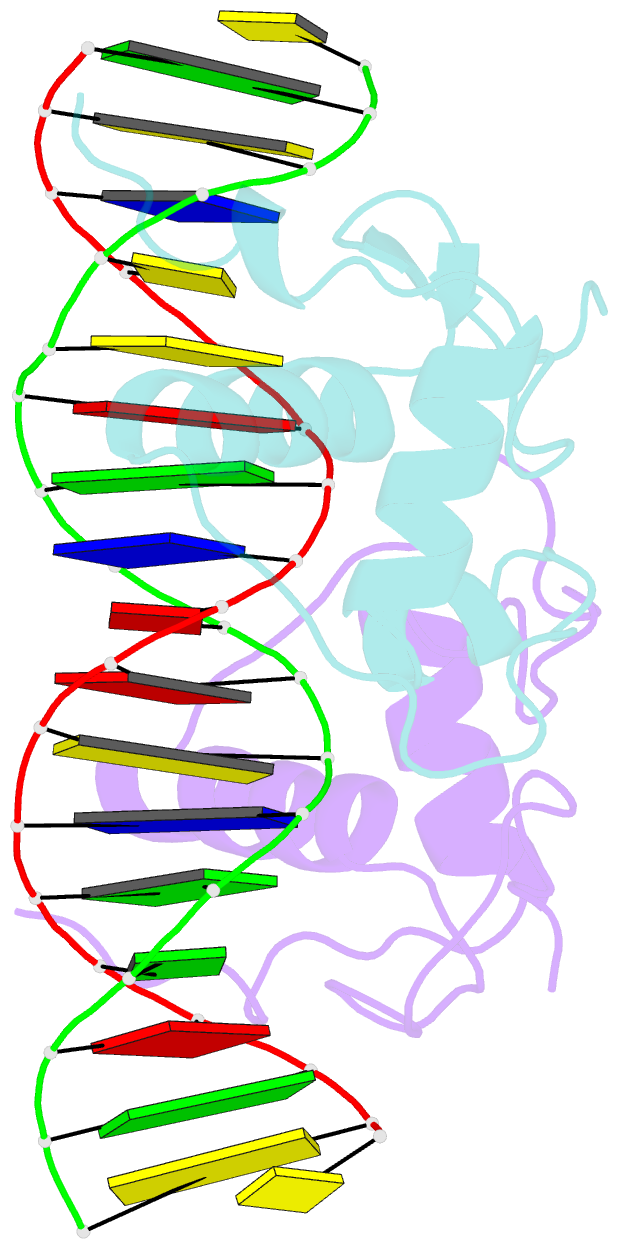
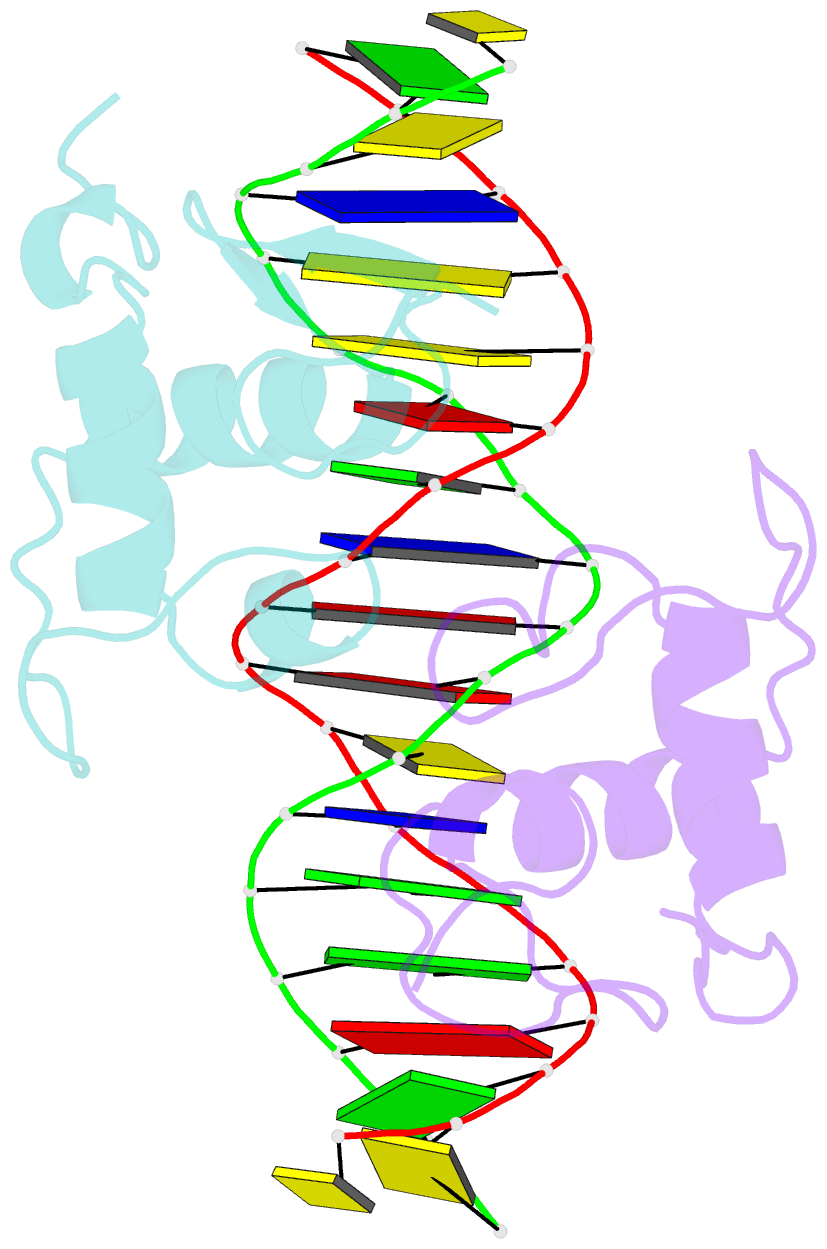
- Crystal structure of the heterodimeric ecdysone receptor DNA-binding complex
- Reference
- Devarakonda S, Harp JM, Kim Y, Ozyhar A, Rastinejad F (2003): "Structure of the Heterodimeric Ecdysone Receptor DNA-binding Complex." Embo J., 22, 5827-5840. doi: 10.1093/emboj/cdg569.
- Abstract
- Ecdysteroids initiate molting and metamorphosis in insects via a heterodimeric receptor consisting of the ecdysone receptor (EcR) and ultraspiracle (USP). The EcR-USP heterodimer preferentially mediates transcription through highly degenerate pseudo-palindromic response elements, resembling inverted repeats of 5'-AGGTCA-3' separated by 1 bp (IR-1). The requirement for a heterodimeric arrangement of EcR-USP subunits to bind to a symmetric DNA is unusual within the nuclear receptor superfamily. We describe the 2.24 A structure of the EcR-USP DNA-binding domain (DBD) heterodimer bound to an idealized IR-1 element. EcR and USP use similar surfaces, and rely on the deformed minor groove of the DNA to establish protein-protein contacts. As retinoid X receptor (RXR) is the mammalian homolog of USP, we also solved the 2.60 A crystal structure of the EcR-RXR DBD heterodimer on IR-1 and found the dimerization and DNA-binding interfaces to be the same as in the EcR-USP complex. Sequence alignments indicate that the EcR-RXR heterodimer is an important model for understanding how the FXR-RXR heterodimer binds to IR-1 sites.