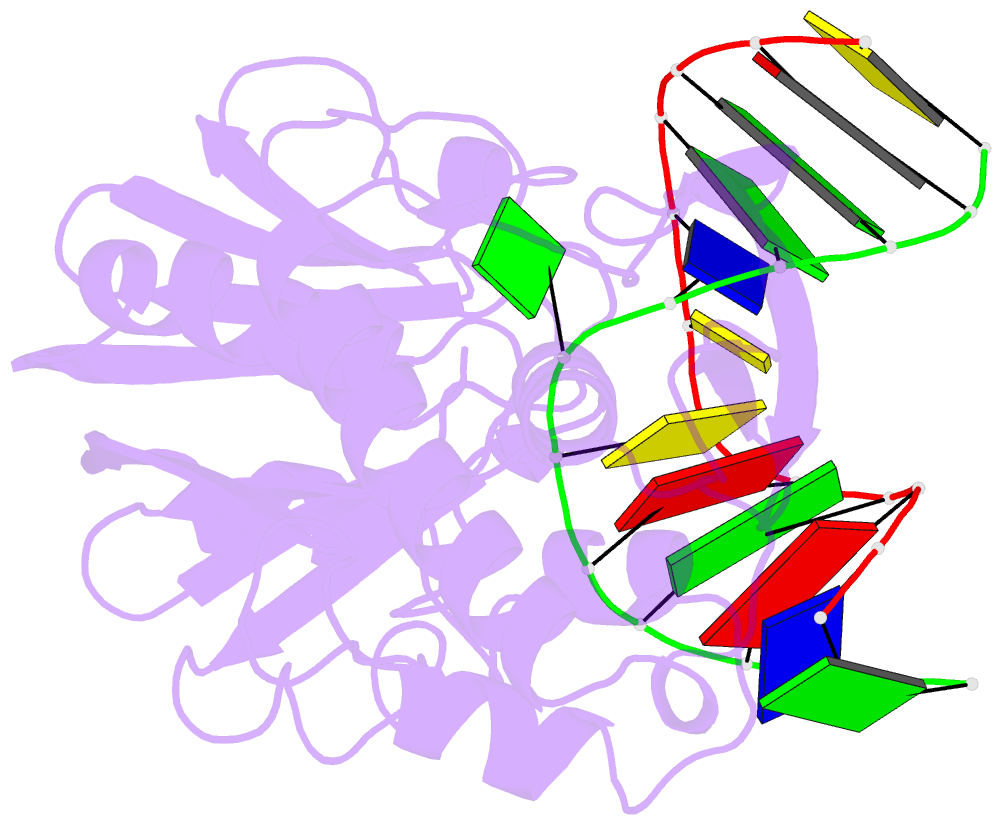
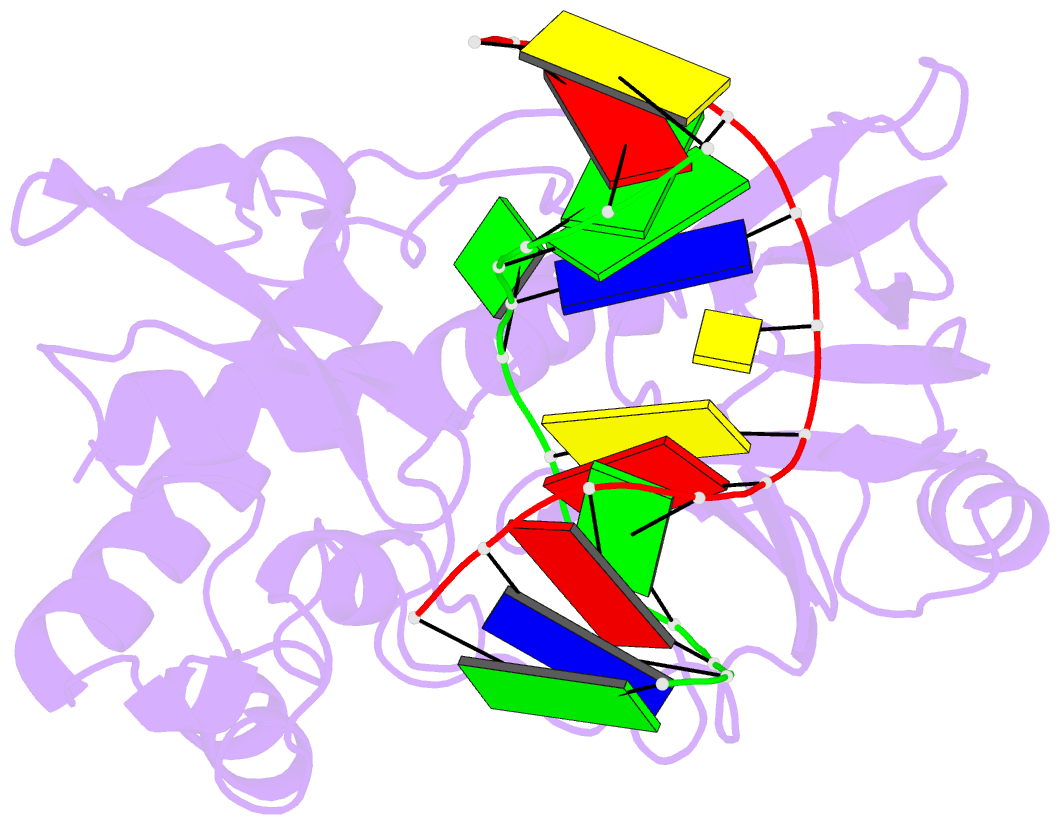
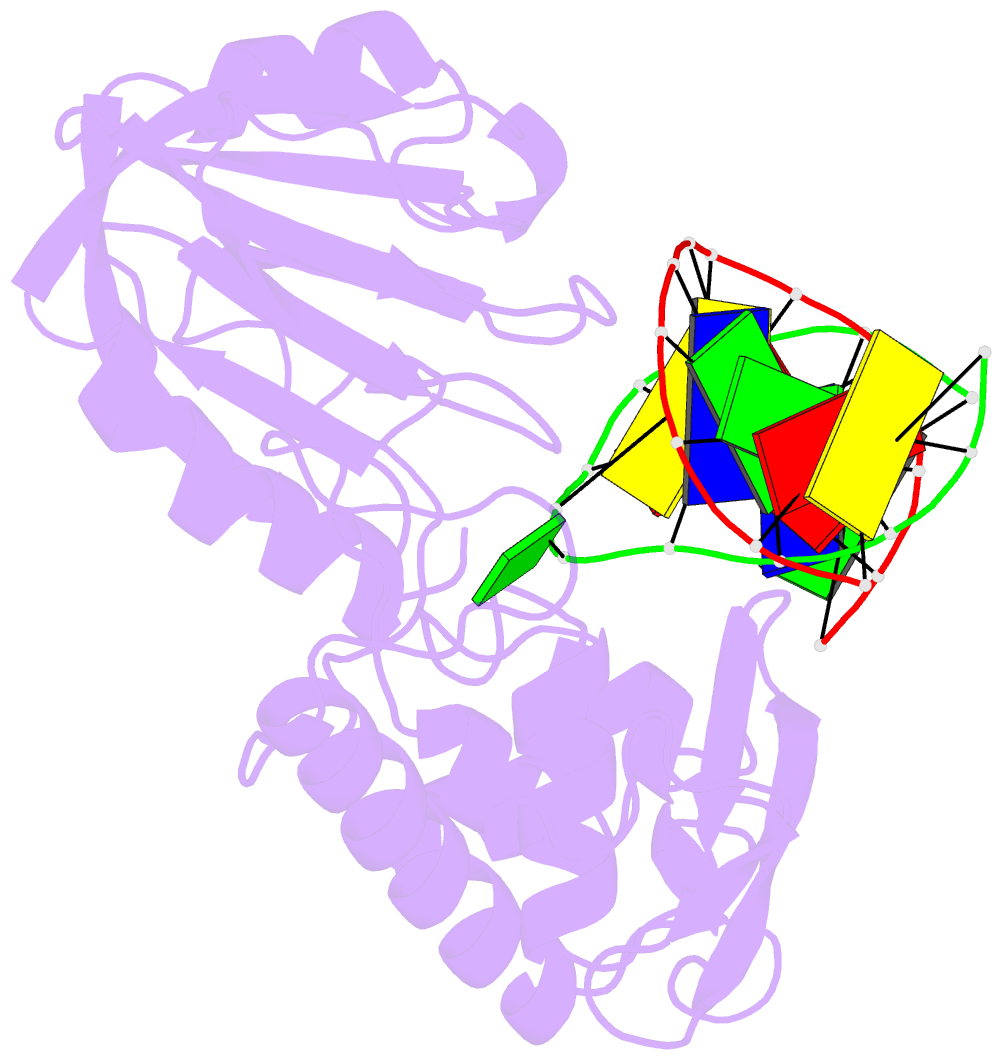
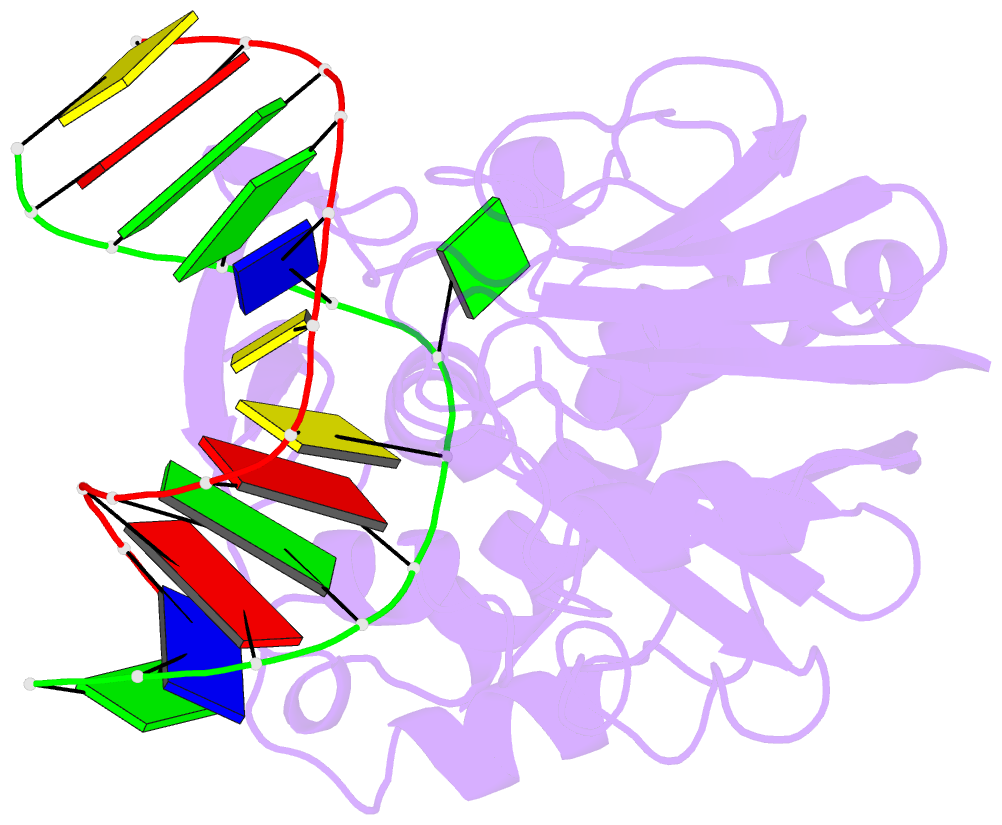
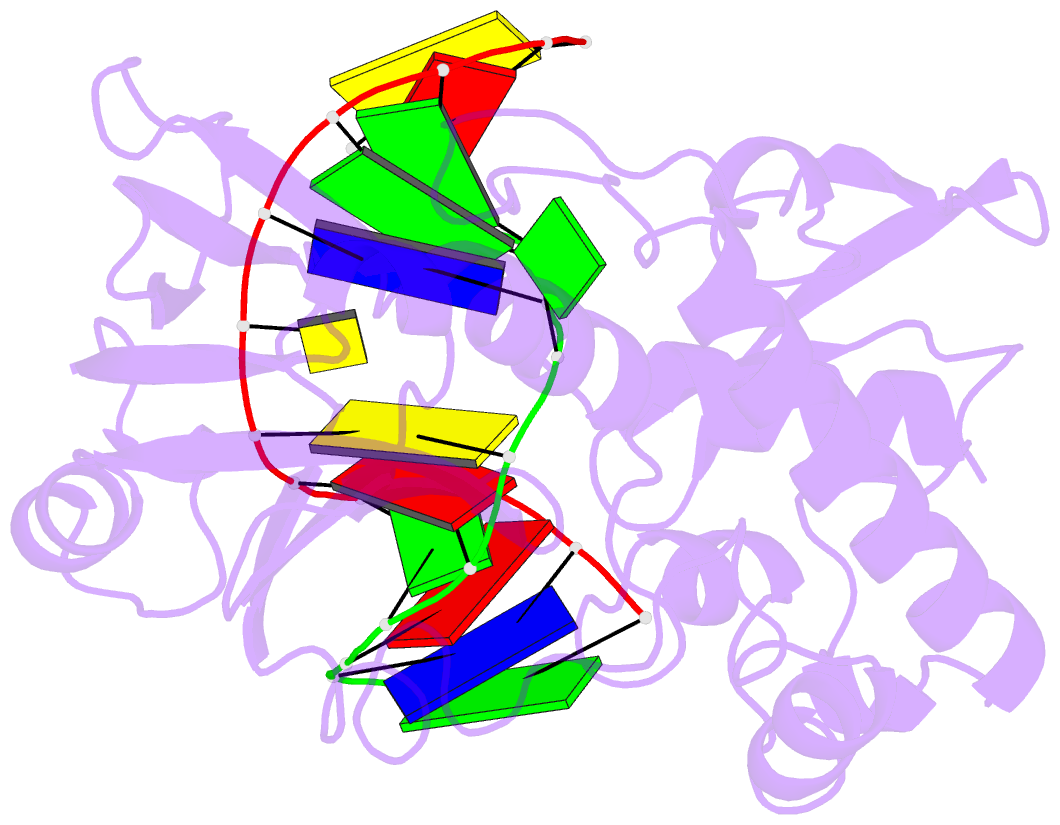
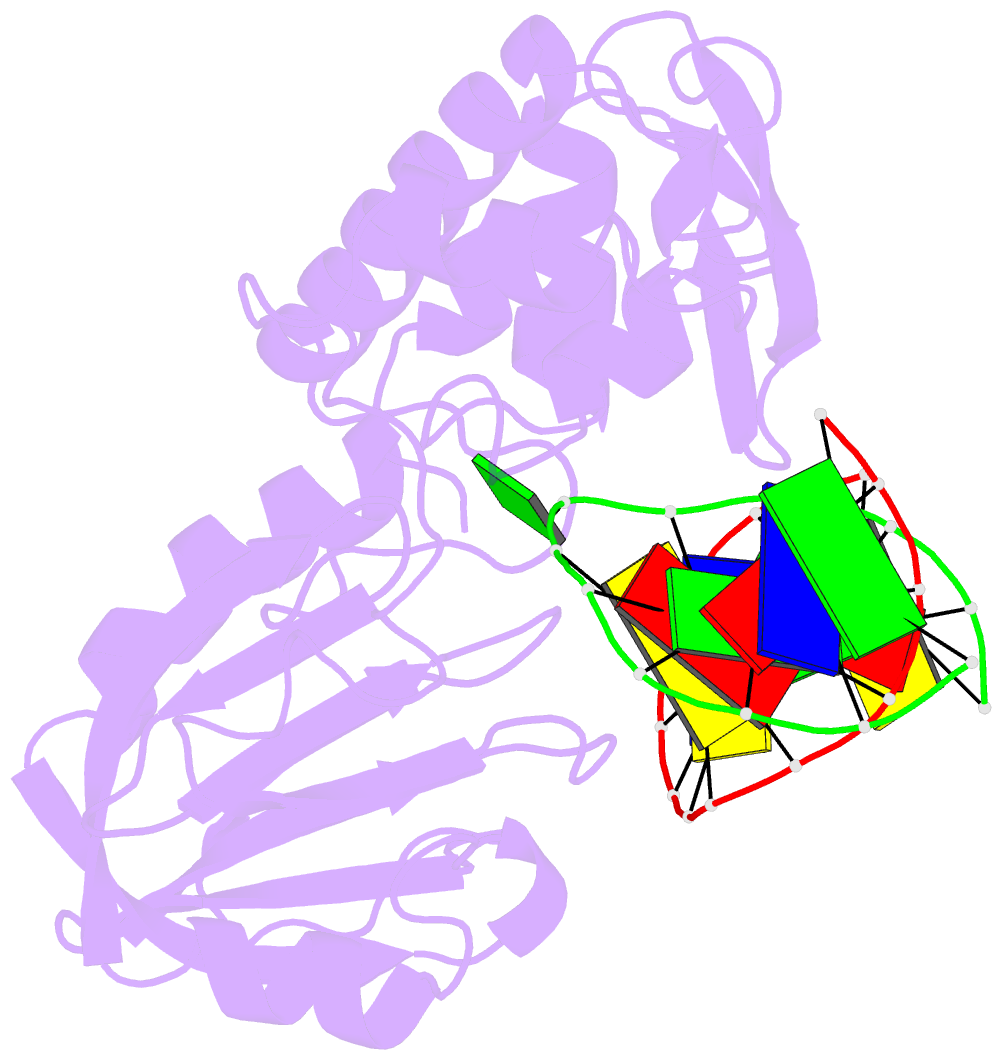
Summary information and primary citation
- PDB-id
- 1r2y; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.34 Å)
- Summary
- Mutm (fpg) bound to 8-oxoguanine (oxog) containing DNA
- Reference
- Fromme JC, Verdine GL (2003): "DNA Lesion Recognition by the Bacterial Repair Enzyme MutM." J.Biol.Chem., 278, 51543-51548. doi: 10.1074/jbc.M307768200.
- Abstract
- MutM is a bacterial DNA glycosylase that removes the mutagenic lesion 8-oxoguanine (oxoG) from duplex DNA. The means of oxoG recognition by MutM (also known as Fpg) is of fundamental interest, in light of the vast excess of normal guanine bases present in genomic DNA. The crystal structure of a recognition-competent but catalytically inactive version of MutM in complex with oxoG-containing DNA reveals the structural basis for recognition. MutM binds the oxoG nucleoside in the syn glycosidic configuration and distinguishes oxoG from guanine by reading out the protonation state of the N7 atom. The segment of MutM principally responsible for oxoG recognition is a flexible loop, suggesting that conformational mobility influences lesion recognition and catalysis. Furthermore, the structure of MutM in complex with DNA containing an alternative substrate, dihydrouracil, demonstrates how MutM is able to recognize lesions other than oxoG.