Summary information and primary citation
- PDB-id
- 1rgo; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- NMR
- Summary
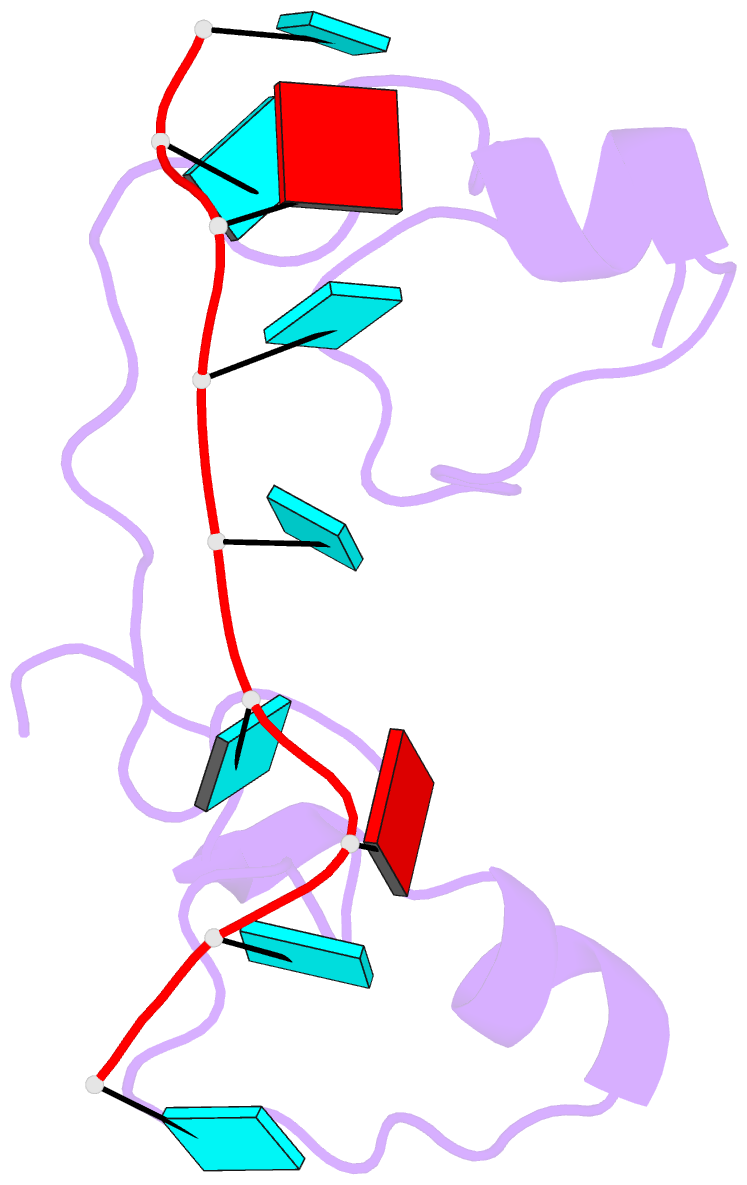
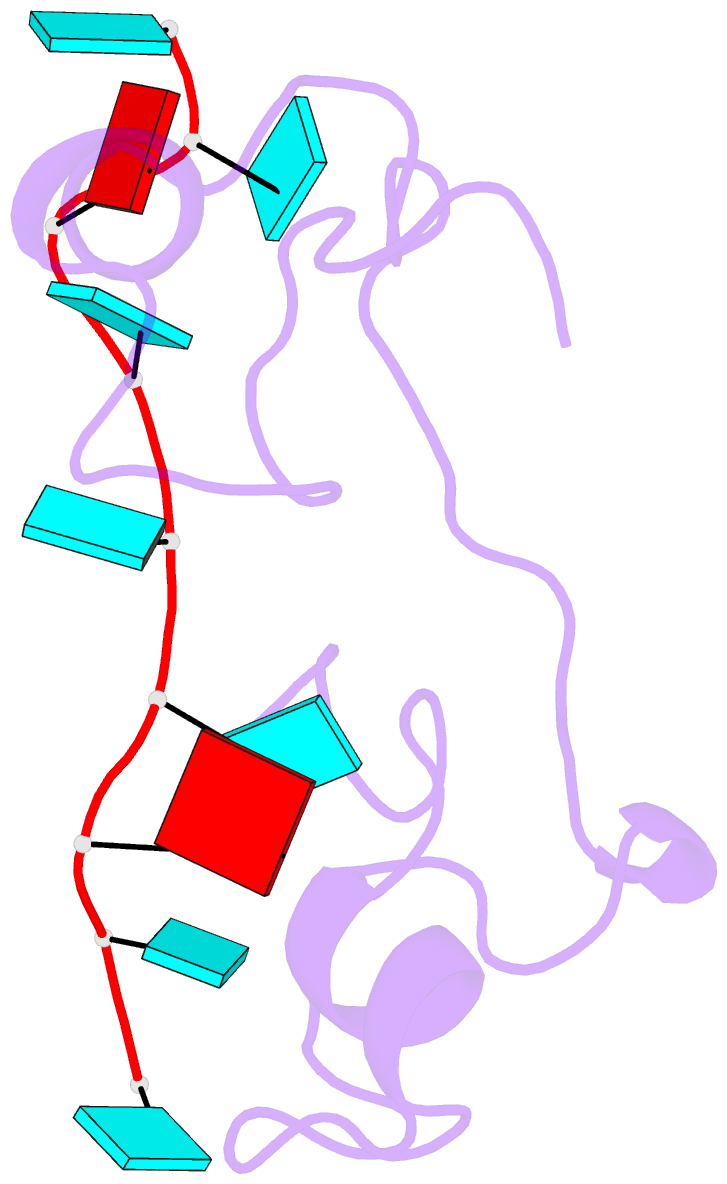
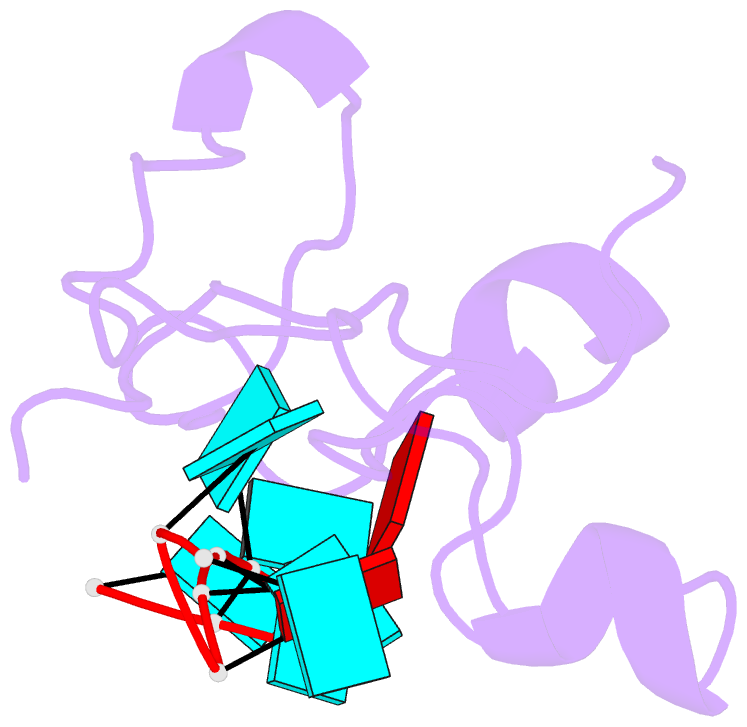
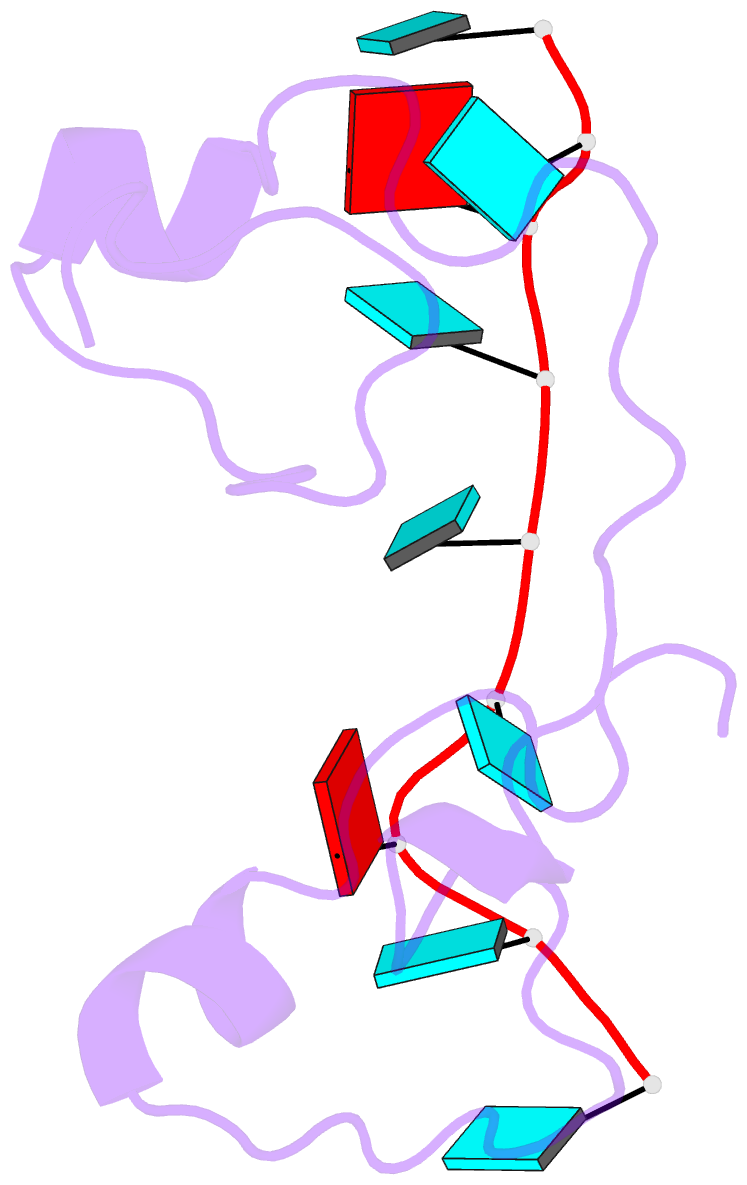
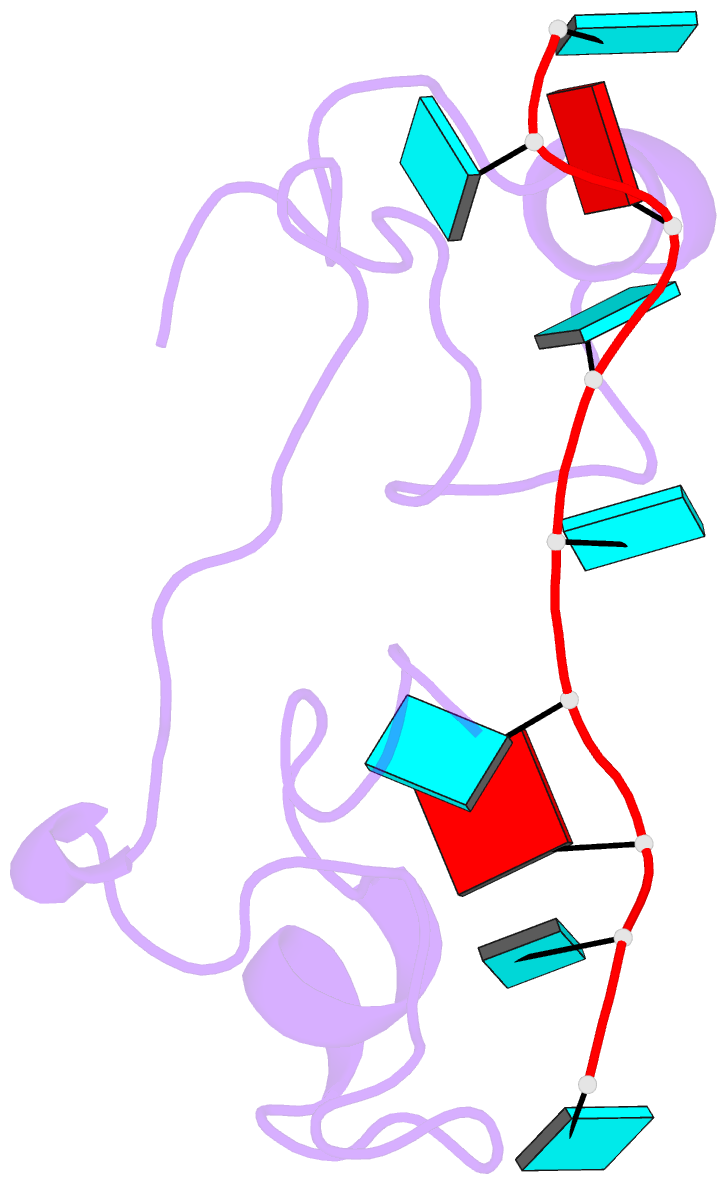
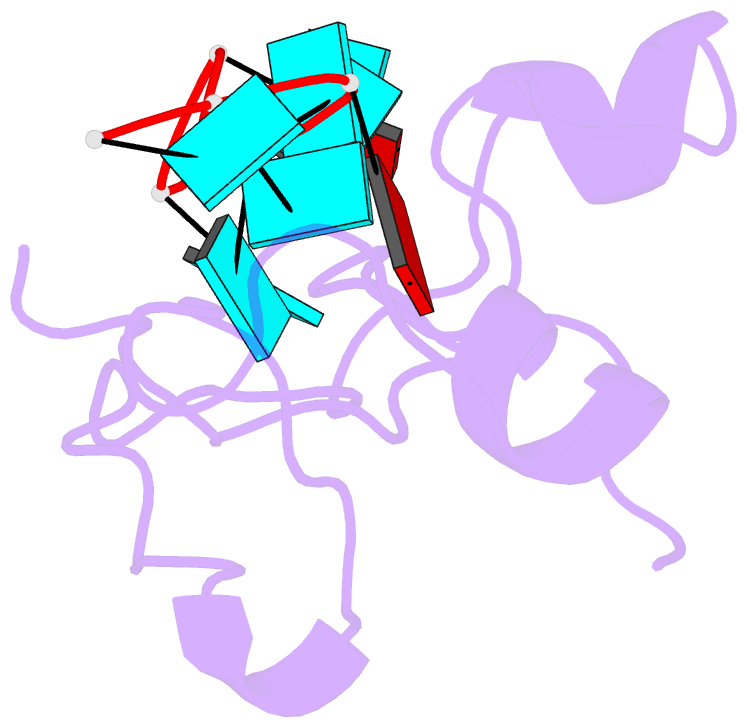
- Structural basis for recognition of the mrna class ii au-rich element by the tandem zinc finger domain of tis11d
- Reference
- Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE (2004): "Recognition of the mRNA AU-rich element by the zinc finger domain of TIS11d." NAT.STRUCT.MOL.BIOL., 11, 257-264. doi: 10.1038/nsmb738.
- Abstract
- The tandem zinc finger (TZF) domain of the protein TIS11d binds to the class II AU-rich element (ARE) in the 3' untranslated region (3' UTR) of target mRNAs and promotes their deadenylation and degradation. The NMR structure of the TIS11d TZF domain bound to the RNA sequence 5'-UUAUUUAUU-3' comprises a pair of novel CCCH fingers of type CX(8)CX(5)CX(3)H separated by an 18-residue linker. The two TIS11d zinc fingers bind in a symmetrical fashion to adjacent 5'-UAUU-3' subsites on the single-stranded RNA via a combination of electrostatic and hydrogen-bonding interactions, with intercalative stacking between conserved aromatic side chains and the RNA bases. Sequence specificity in RNA recognition is achieved by a network of intermolecular hydrogen bonds, mostly between TIS11d main-chain functional groups and the Watson-Crick edges of the bases. The TIS11d structure provides insights into the RNA-binding functions of this large family of CCCH zinc finger proteins.