Summary information and primary citation
- PDB-id
- 1rzr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-transport protein-DNA
- Method
- X-ray (2.8 Å)
- Summary
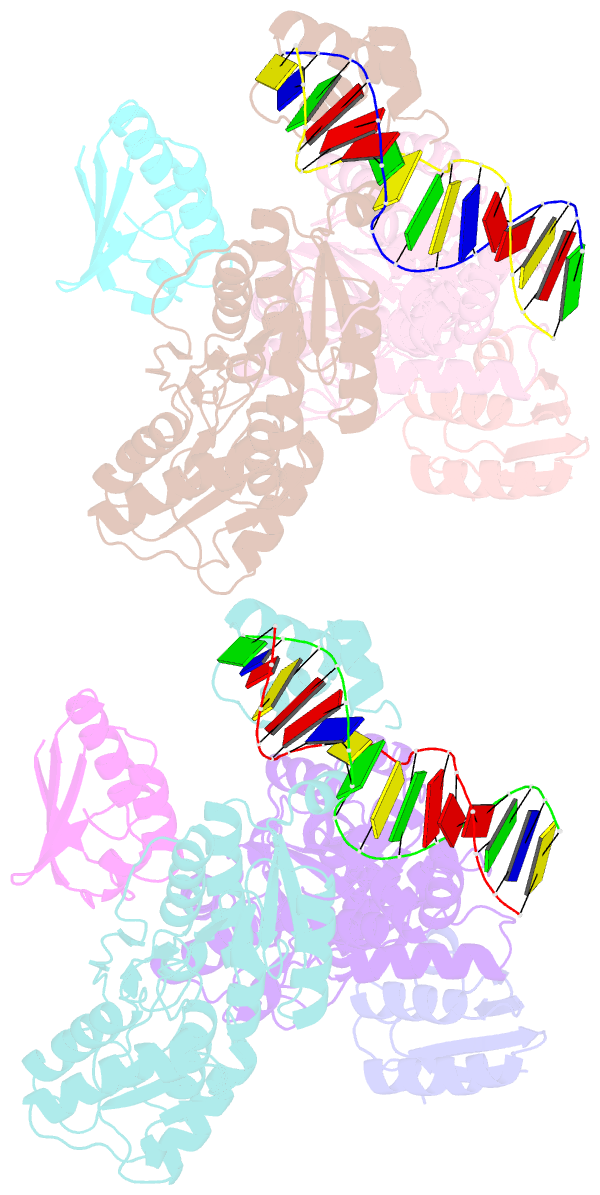
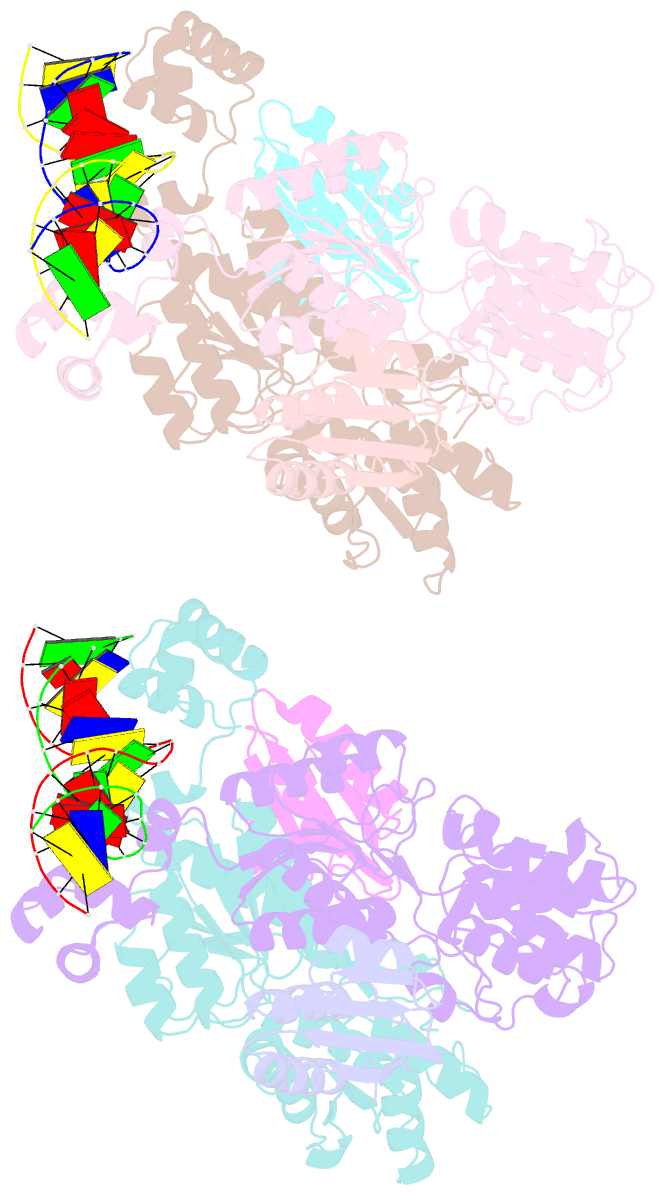
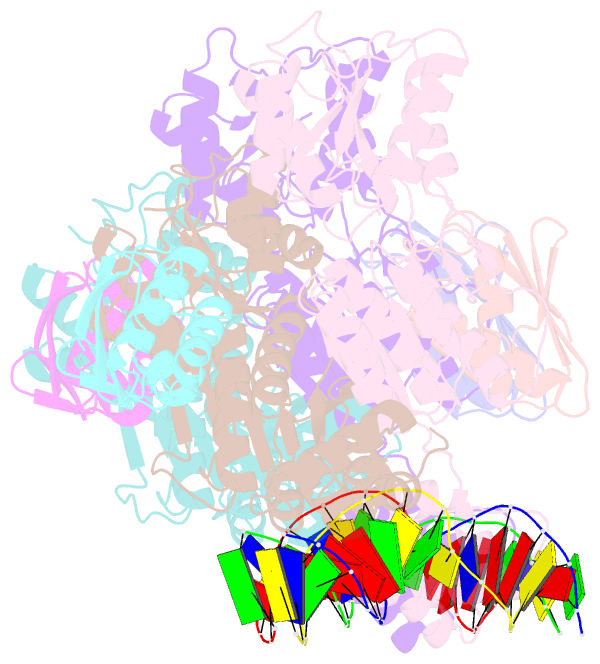
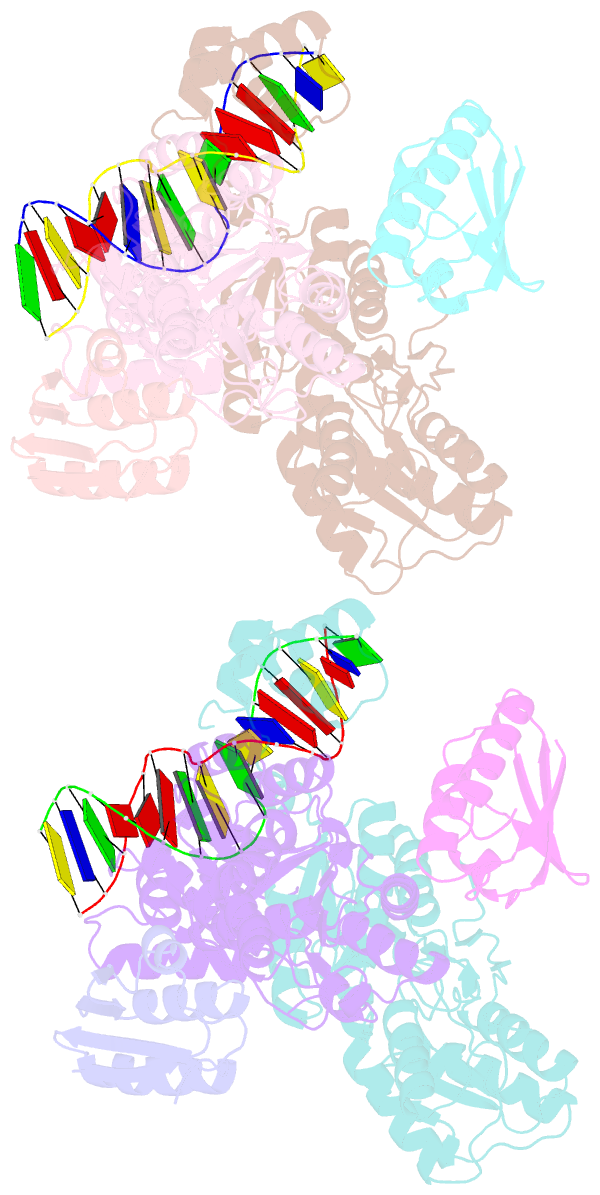
- Crystal structure of transcriptional regulator-phosphoprotein-DNA complex
- Reference
- Schumacher MA, Allen GS, Diel M, Seidel G, Hillen W, Brennan RG (2004): "Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P." Cell(Cambridge,Mass.), 118, 731-741. doi: 10.1016/j.cell.2004.08.027.
- Abstract
- Carbon catabolite repression (CCR) is one of the most fundamental environmental-sensing mechanisms in bacteria and imparts competitive advantage by establishing priorities in carbon metabolism. In gram-positive bacteria, the master transcription regulator of CCR is CcpA. CcpA is a LacI-GalR family member that employs, as an allosteric corepressor, the phosphoprotein HPr-Ser46-P, which is formed in glucose-replete conditions. Here we report structures of the Bacillus megaterium apoCcpA and a CcpA-(HPr-Ser46-P)-DNA complex. These structures reveal that HPr-Ser46-P mediates a novel two-component allosteric DNA binding activation mechanism that involves both rotation of the CcpA subdomains and relocation of pivot-point residue Thr61, which leads to juxtaposition of the DNA binding regions permitting "hinge" helix formation in the presence of cognate DNA. The structure of the CcpA-(HPr-Ser46-P)-cre complex also reveals the elegant mechanism by which CcpA family-specific interactions with HPr-Ser46-P residues Ser46-P and His15 partition the high-energy CCR and low-energy PTS pathways, the latter requiring HPr-His15-P.