Summary information and primary citation
- PDB-id
- 1si3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-RNA
- Method
- X-ray (2.6 Å)
- Summary
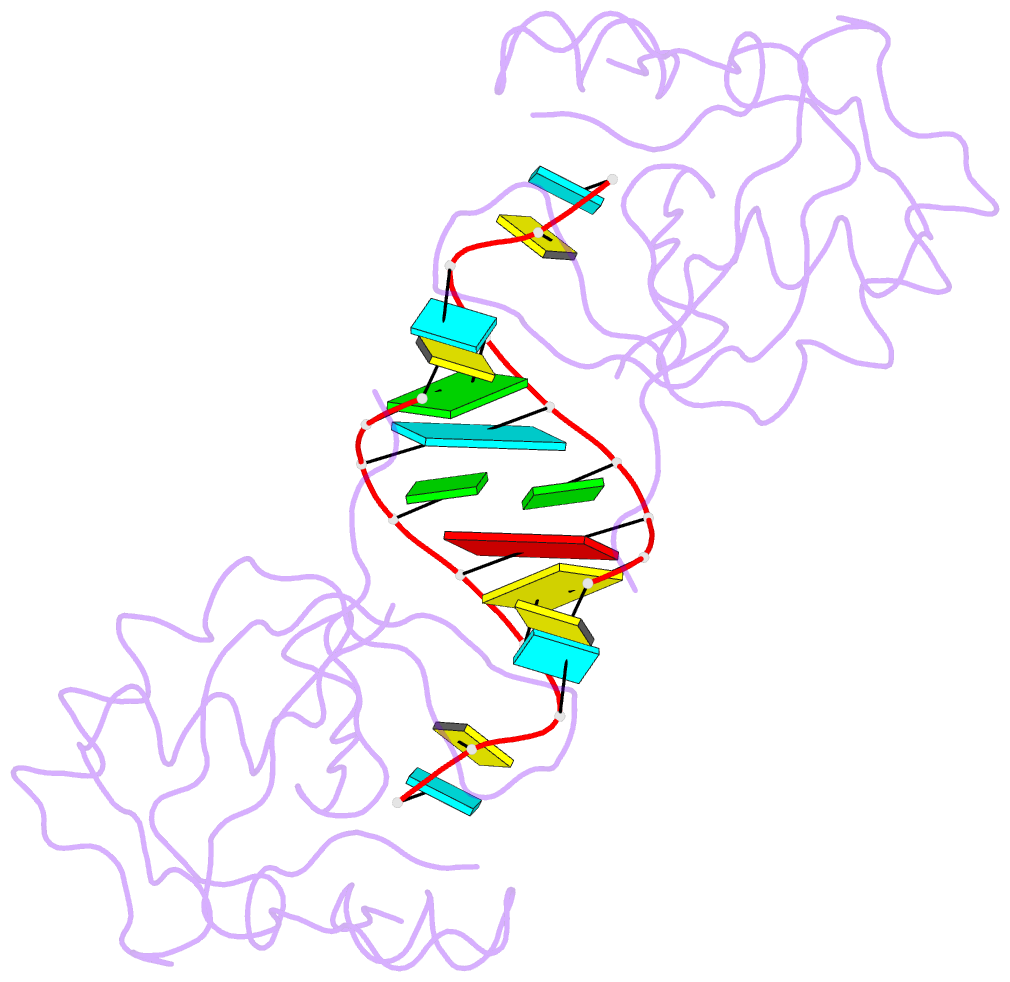
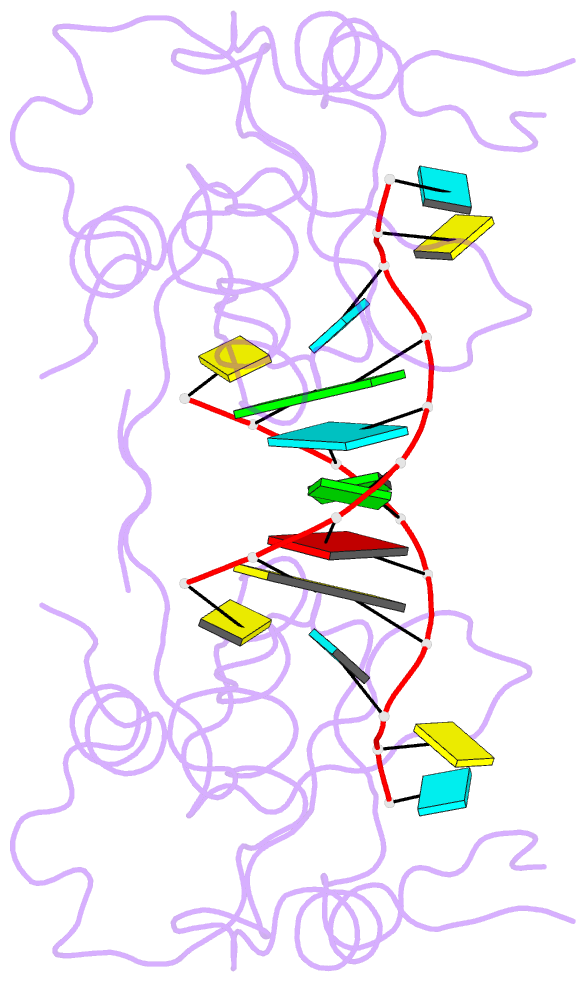
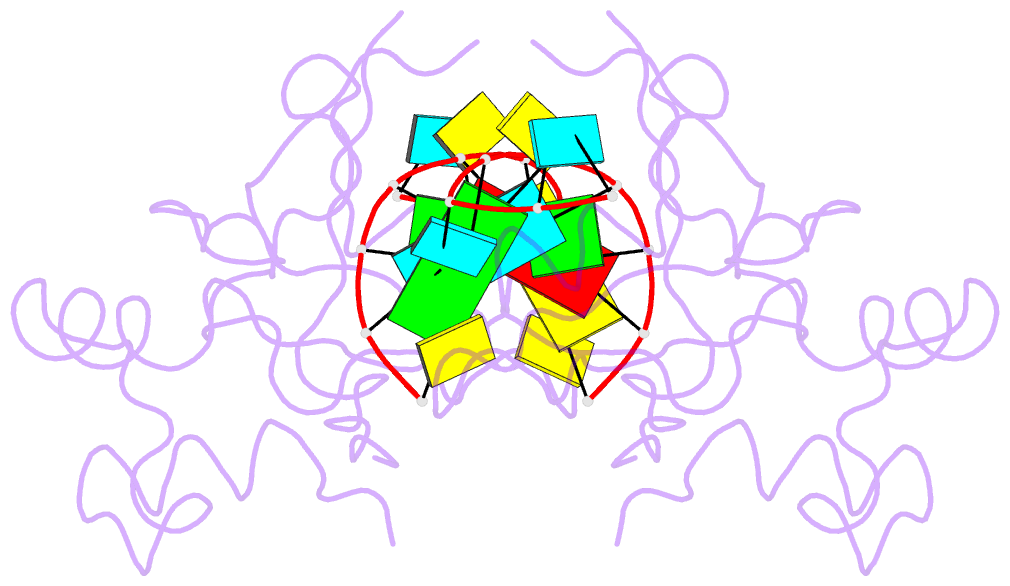
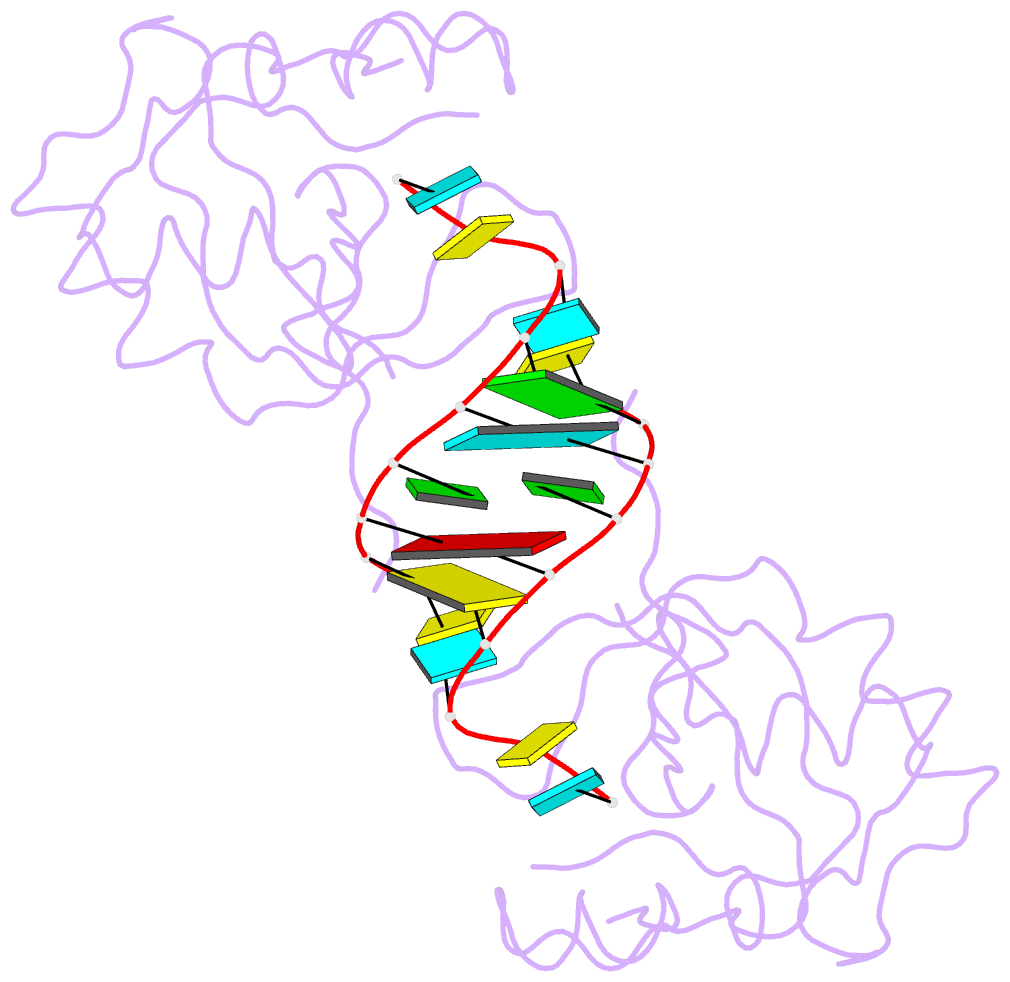
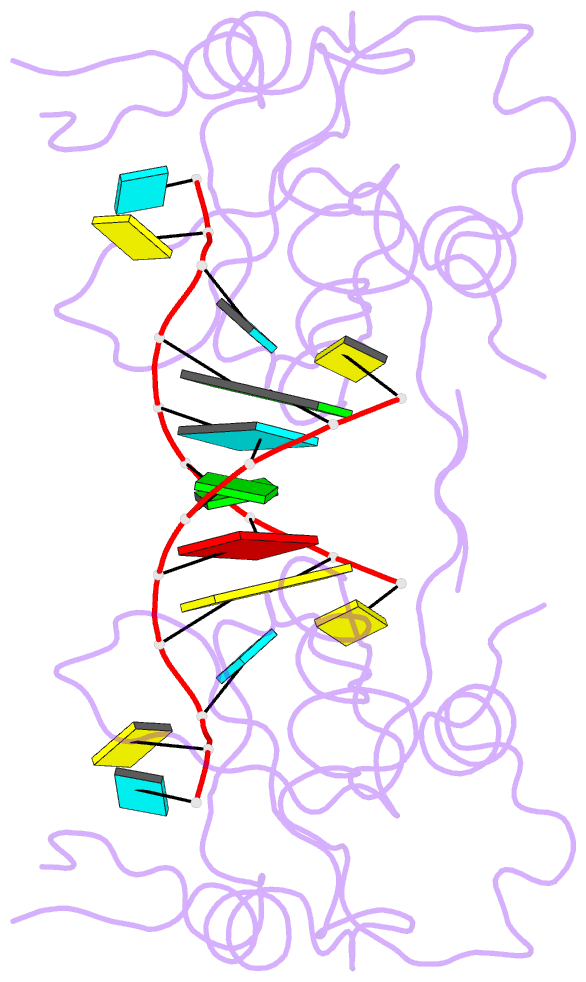
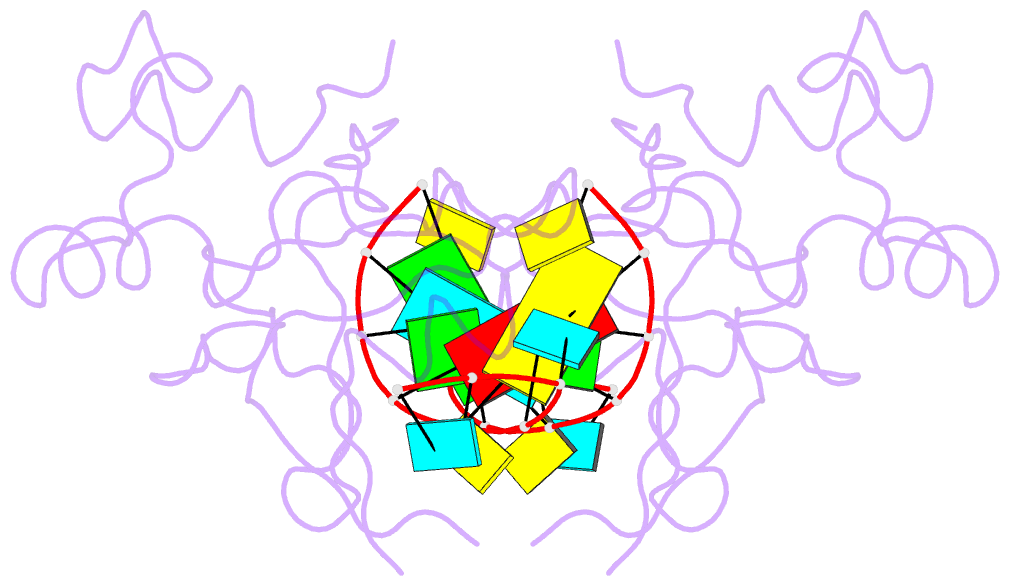
- Crystal structure of the paz domain of human eif2c1 in complex with a 9-mer sirna-like duplex
- Reference
- Ma JB, Ye K, Patel DJ (2004): "Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain." Nature, 429, 318-322. doi: 10.1038/nature02519.
- Abstract
- Short RNAs mediate gene silencing, a process associated with virus resistance, developmental control and heterochromatin formation in eukaryotes. RNA silencing is initiated through Dicer-mediated processing of double-stranded RNA into small interfering RNA (siRNA). The siRNA guide strand associates with the Argonaute protein in silencing effector complexes, recognizes complementary sequences and targets them for silencing. The PAZ domain is an RNA-binding module found in Argonaute and some Dicer proteins and its structure has been determined in the free state. Here, we report the 2.6 A crystal structure of the PAZ domain from human Argonaute eIF2c1 bound to both ends of a 9-mer siRNA-like duplex. In a sequence-independent manner, PAZ anchors the 2-nucleotide 3' overhang of the siRNA-like duplex within a highly conserved binding pocket, and secures the duplex by binding the 7-nucleotide phosphodiester backbone of the overhang-containing strand and capping the 5'-terminal residue of the complementary strand. On the basis of the structure and on binding assays, we propose that PAZ might serve as an siRNA-end-binding module for siRNA transfer in the RNA silencing pathway, and as an anchoring site for the 3' end of guide RNA within silencing effector complexes.