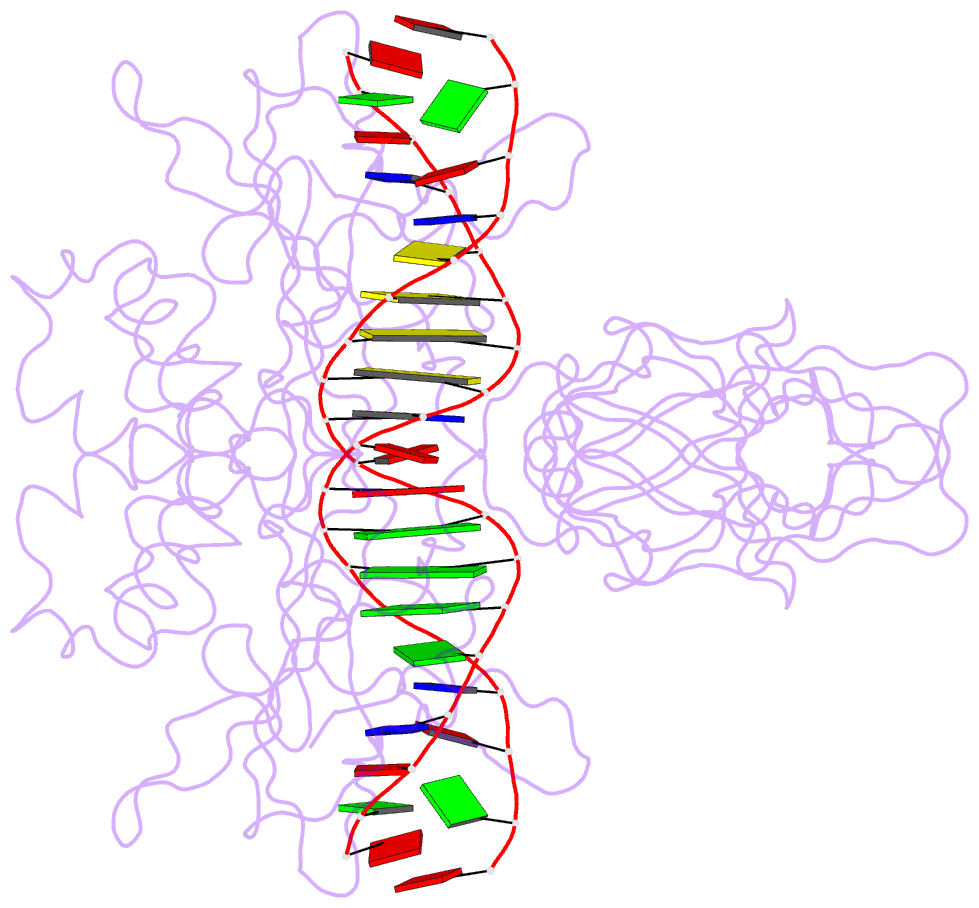
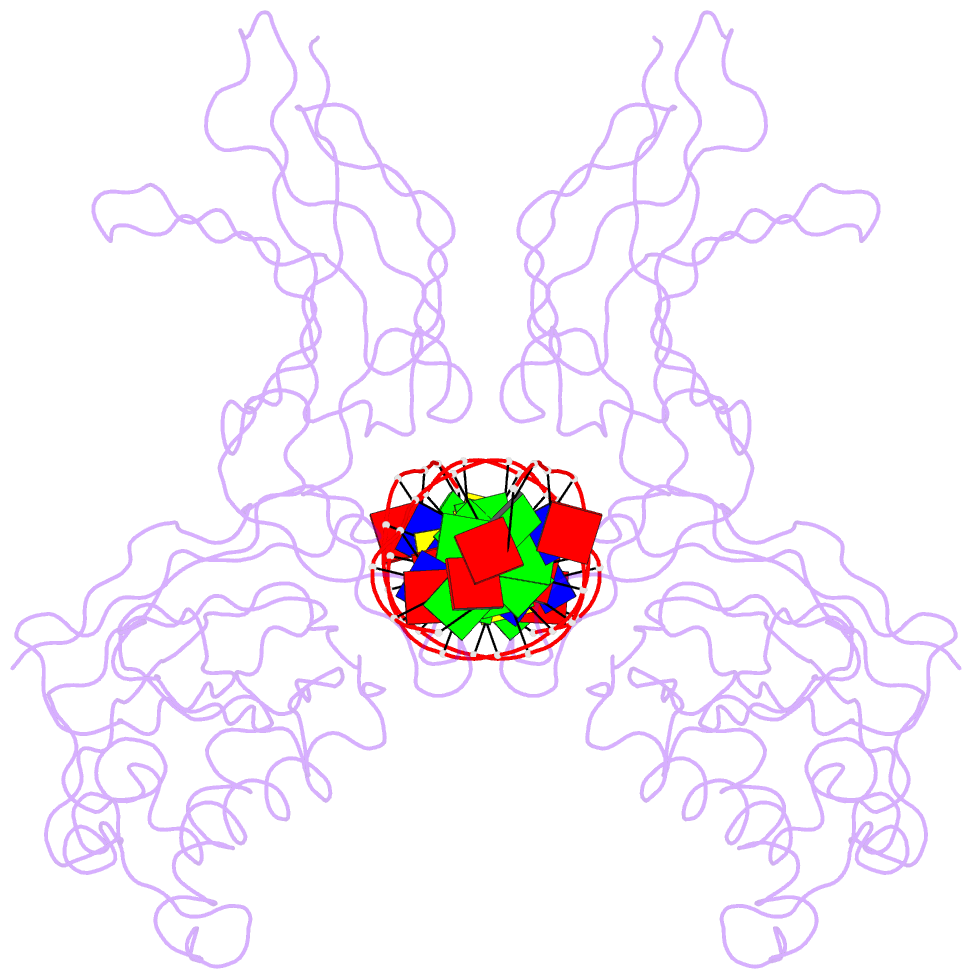
Summary information and primary citation
- PDB-id
- 1svc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.6 Å)
- Summary
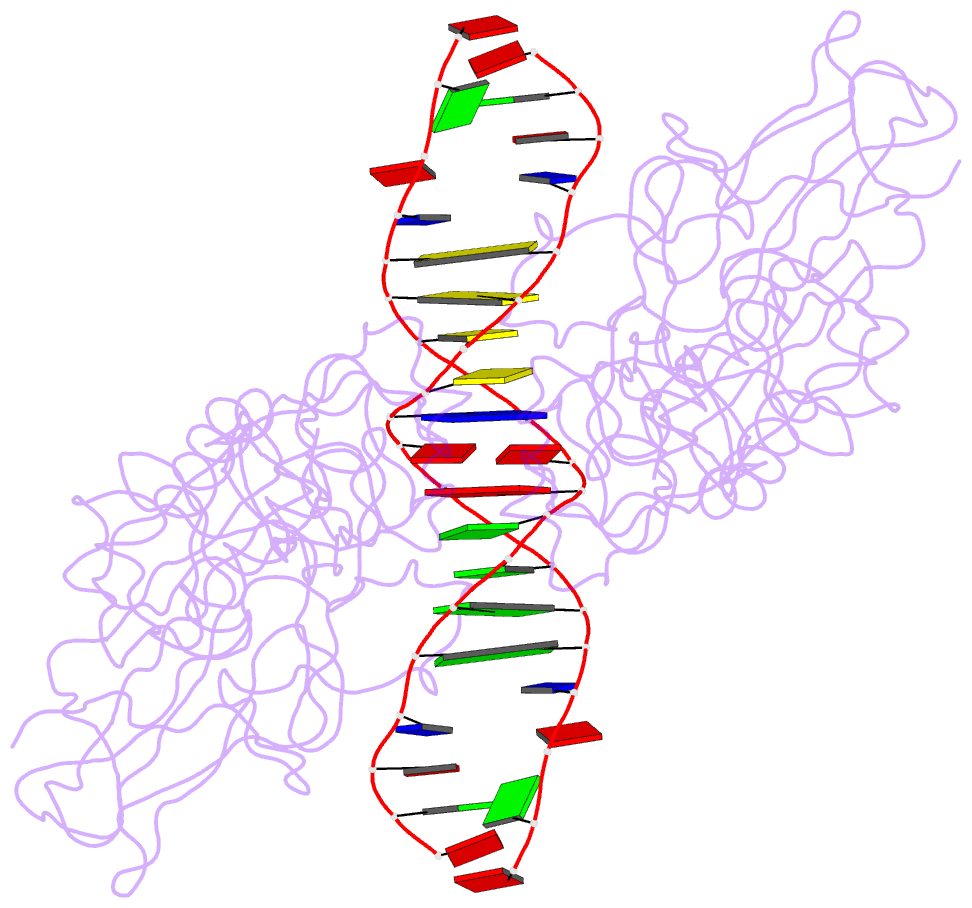
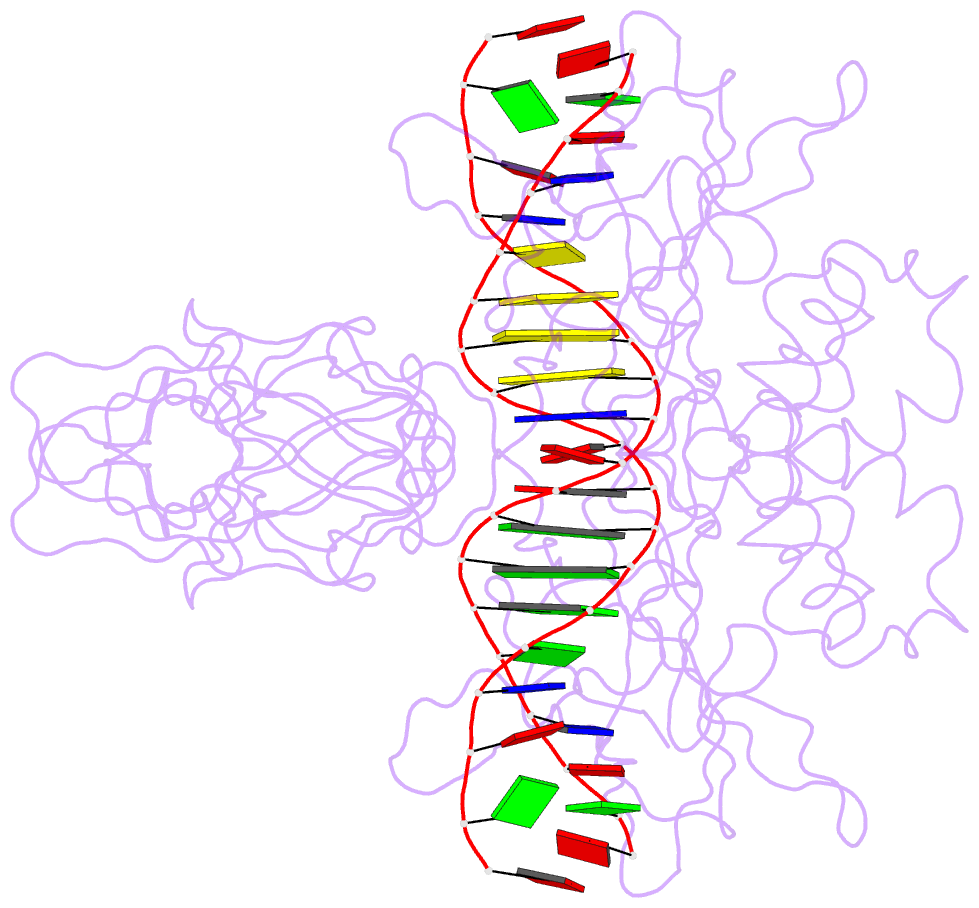
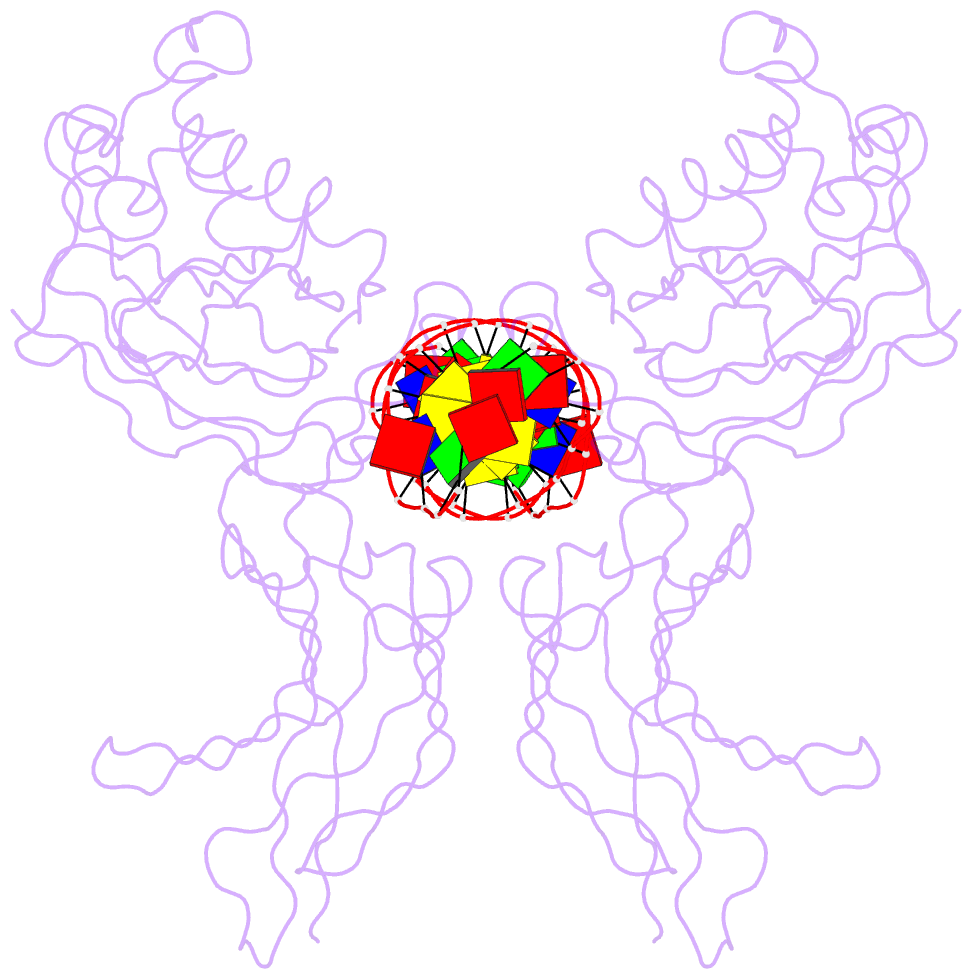
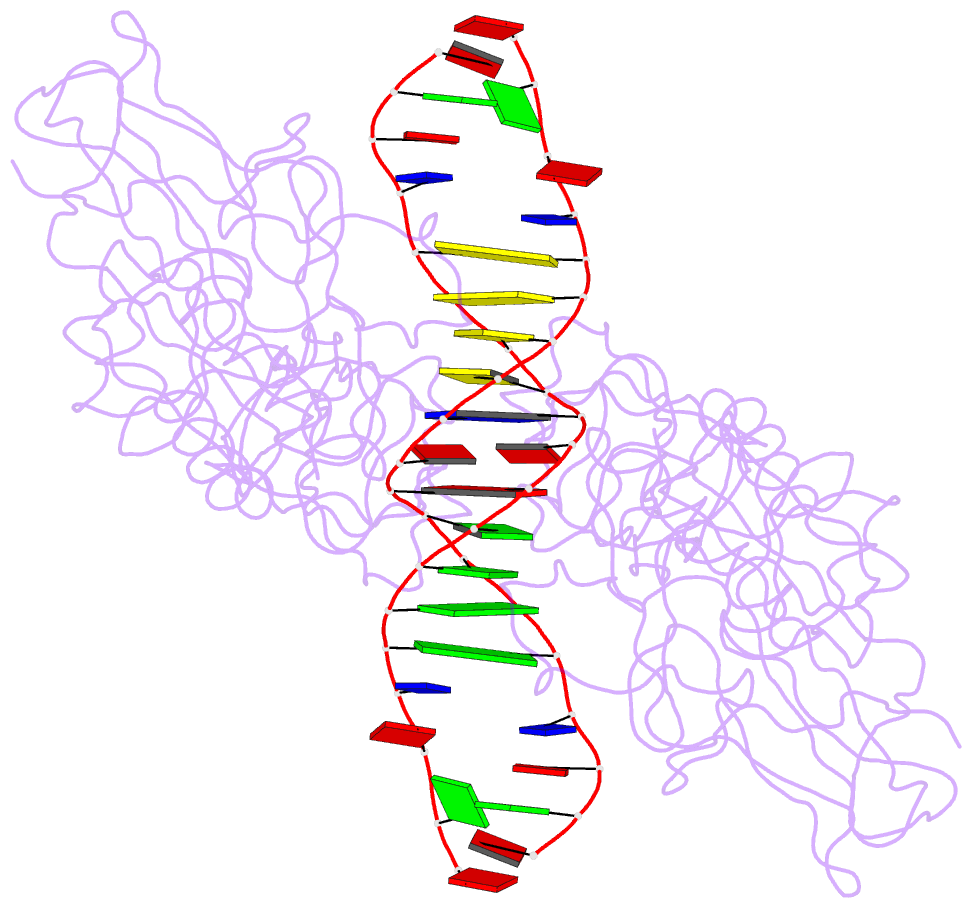
- Nfkb p50 homodimer bound to DNA
- Reference
- Muller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC (1995): "Structure of the NF-kappa B p50 homodimer bound to DNA." Nature, 373, 311-317. doi: 10.1038/373311a0.
- Abstract
- The structure of a large fragment of the p50 subunit of the human transcription factor NF-kappa B, bound as a homodimer to DNA, reveals that the Rel-homology region has two beta-barrel domains that grip DNA in the major groove. Both domains contact the DNA backbone. The amino-terminal specificity domain contains a recognition loop that interacts with DNA bases; the carboxy-terminal dimerization domain bears the site of I-kappa B interaction. The folds of these domains are related to immunoglobulin-like modules. The amino-terminal domain also resembles the core domain of p53.