Summary information and primary citation
- PDB-id
- 1u0b; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- X-ray (2.3 Å)
- Summary
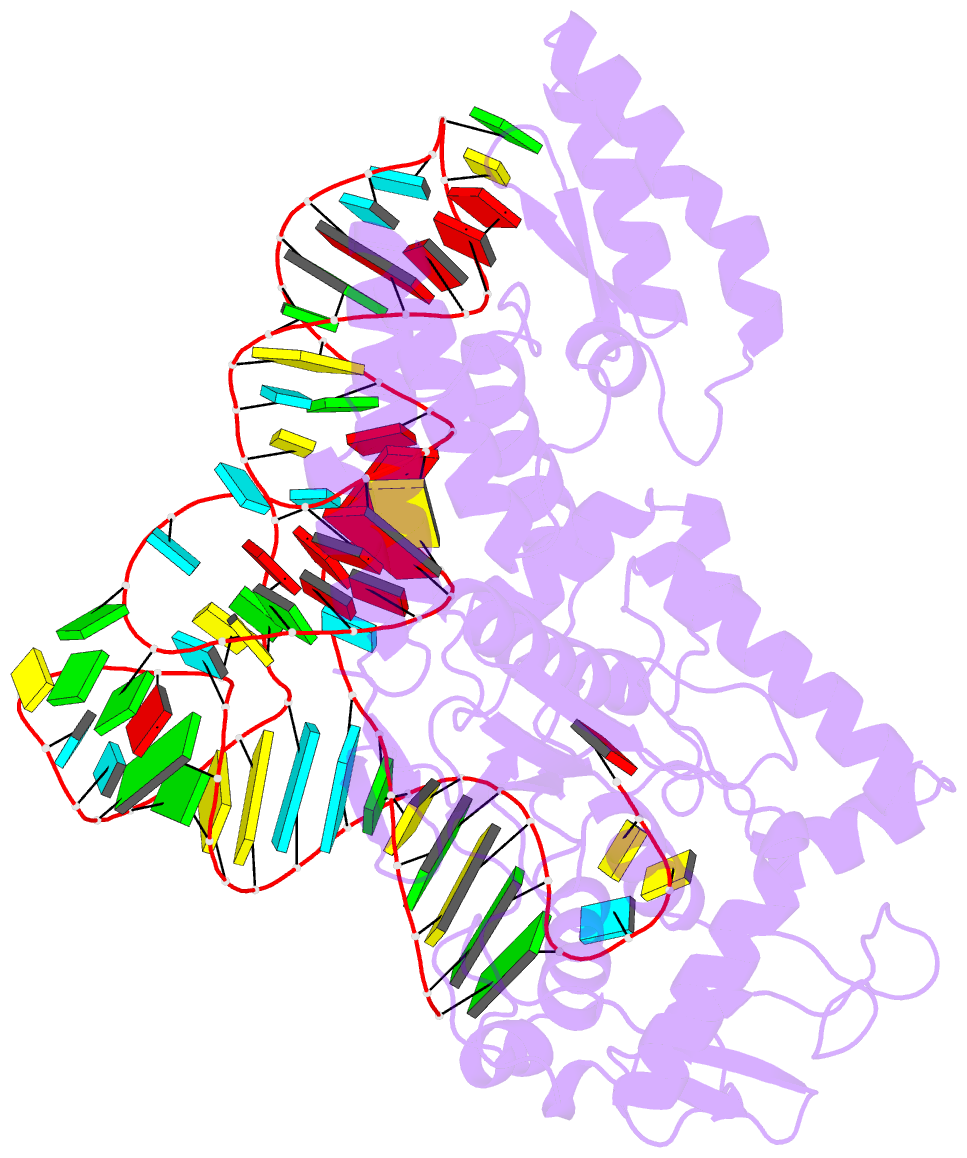
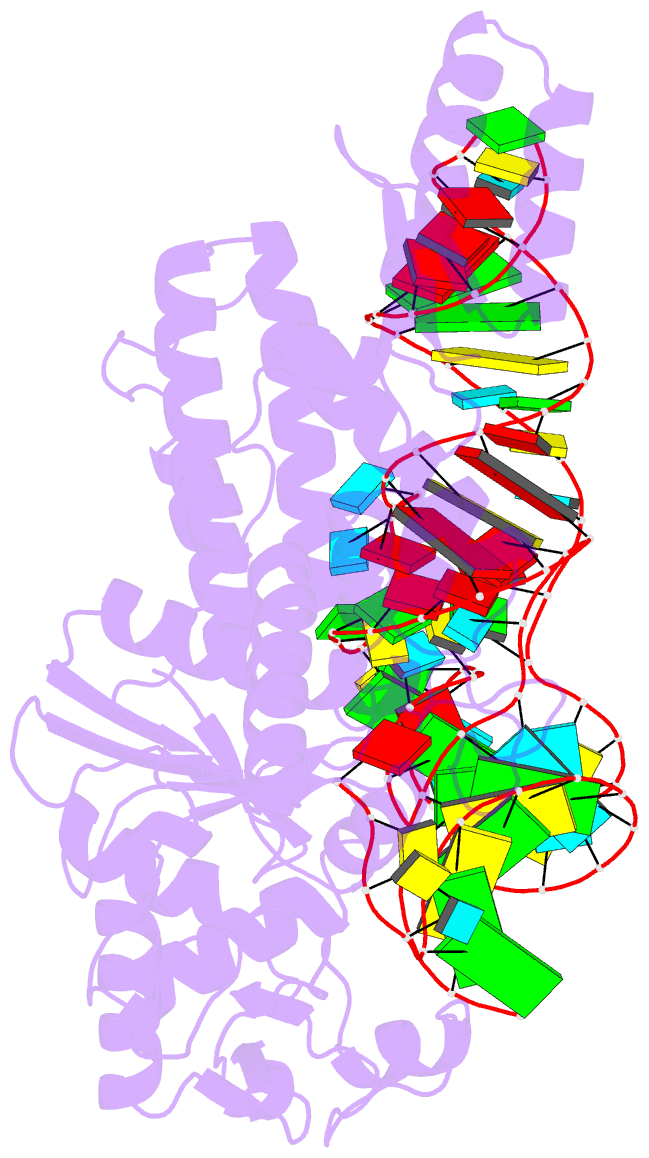
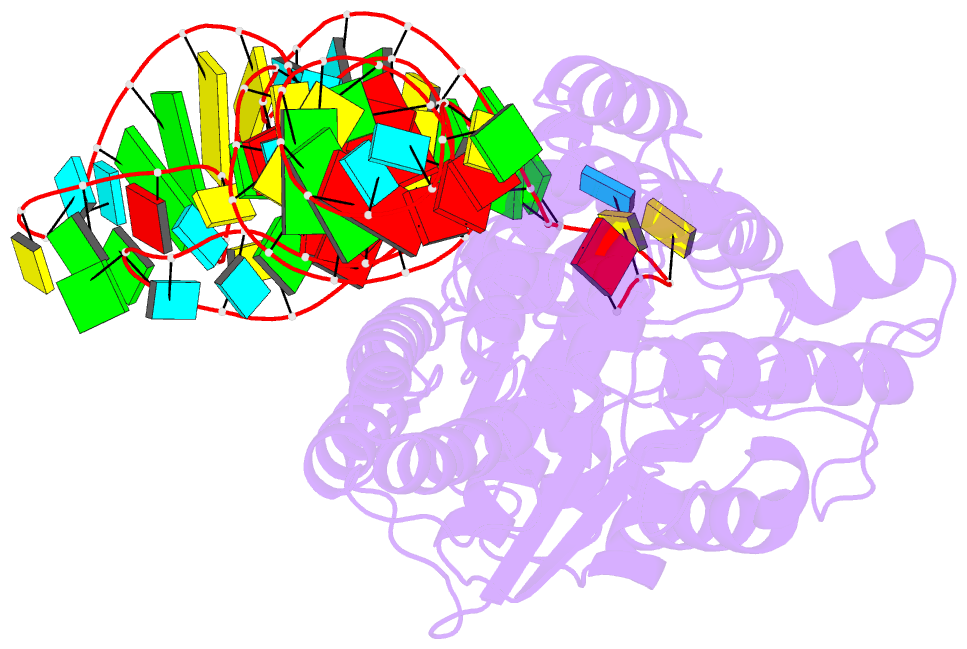
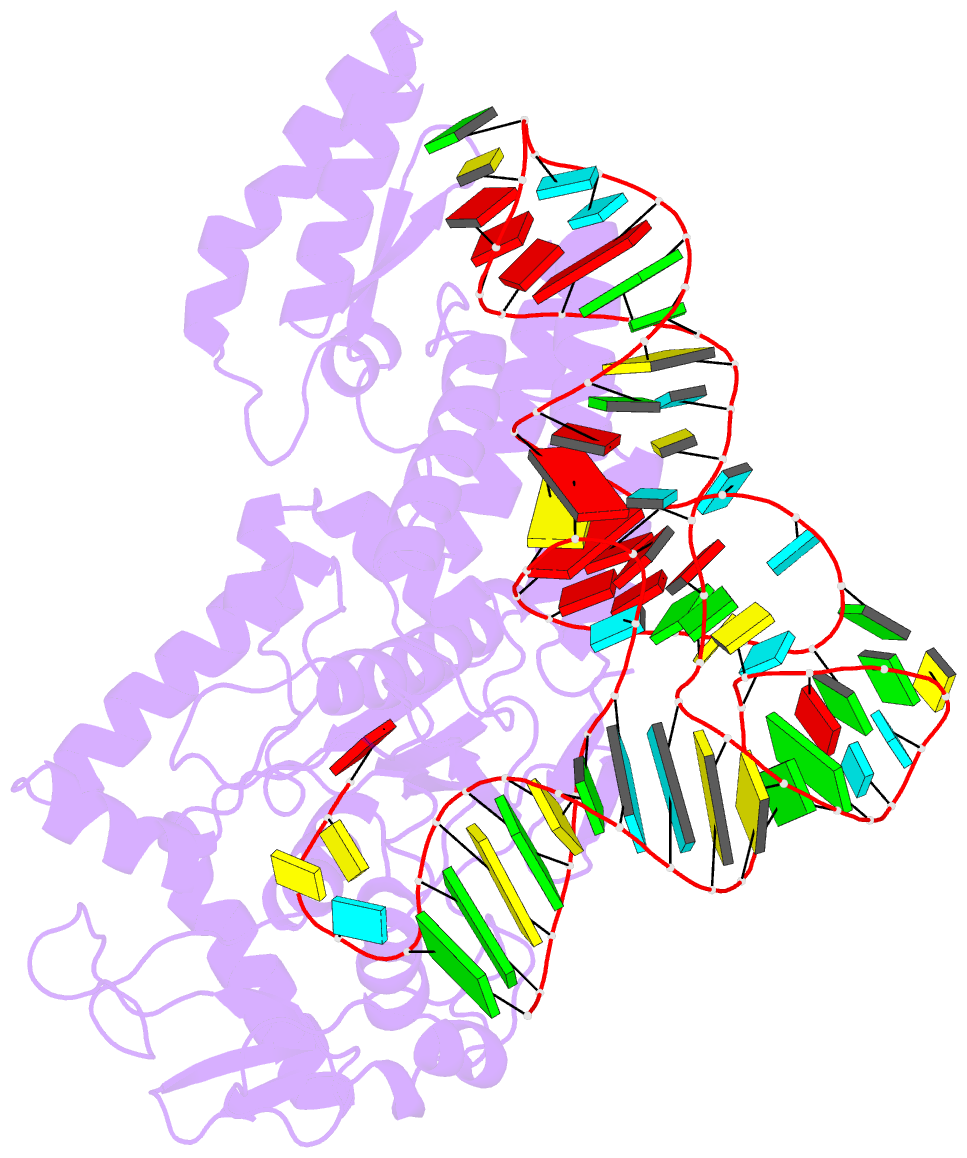
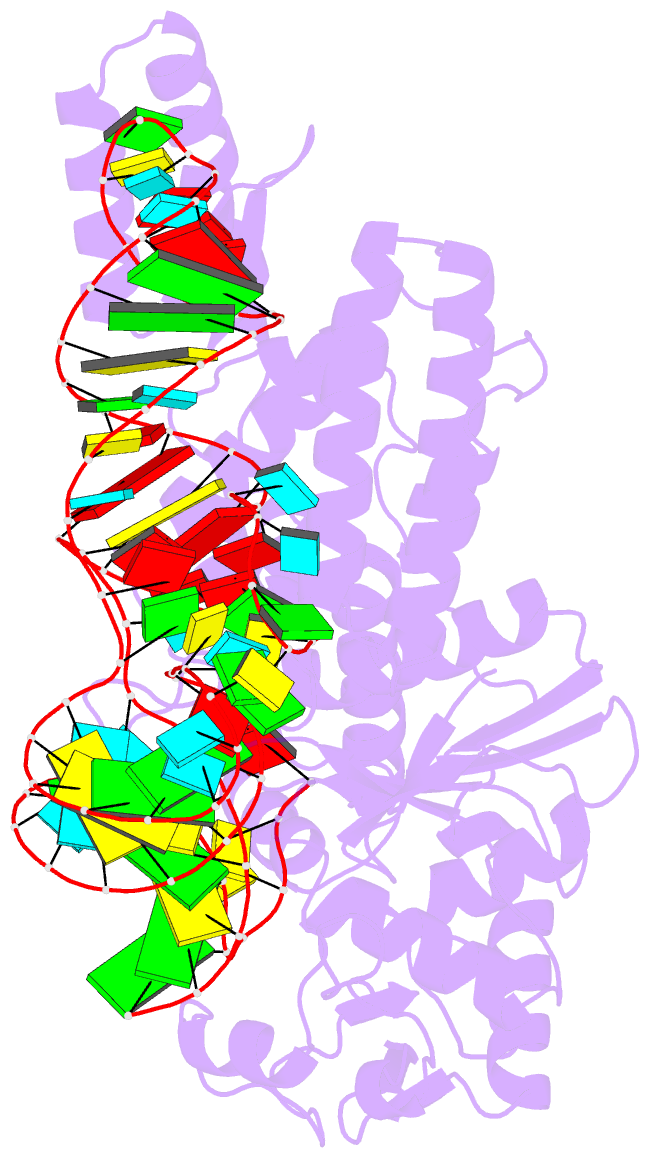
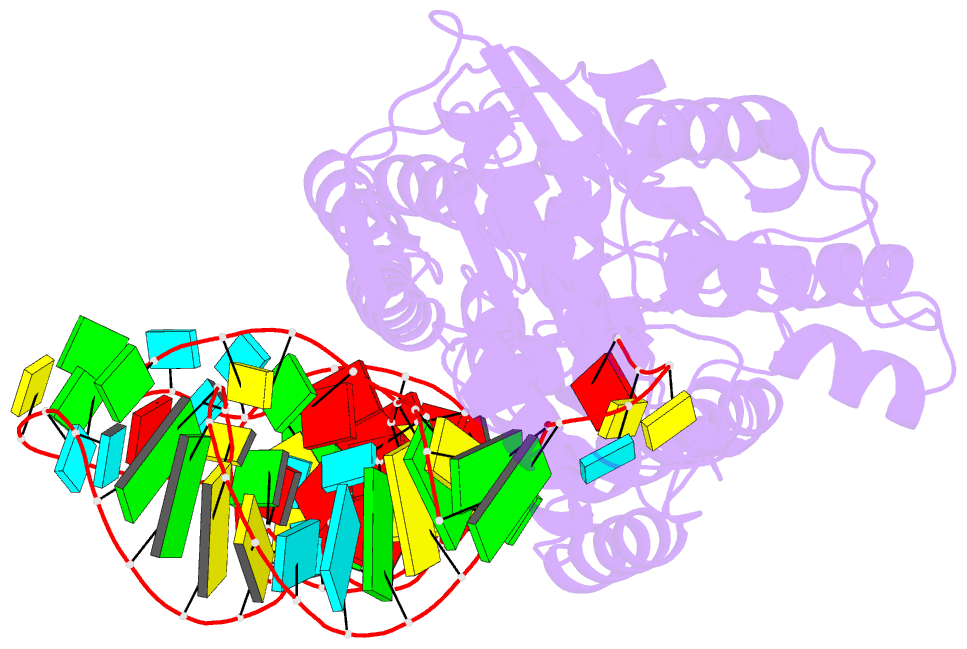
- Crystal structure of cysteinyl-trna synthetase binary complex with trnacys
- Reference
- Hauenstein S, Zhang CM, Hou YM, Perona JJ (2004): "Shape-selective RNA recognition by cysteinyl-tRNA synthetase." Nat.Struct.Mol.Biol., 11, 1134-1141. doi: 10.1038/nsmb849.
- Abstract
- The crystal structure of Escherichia coli cysteinyl-tRNA synthetase (CysRS) bound to tRNA(Cys) at a resolution of 2.3 A reveals base-specific and shape-selective interactions across an extensive protein-RNA recognition interface. The complex contains a mixed alpha/beta C-terminal domain, which is disordered in the unliganded enzyme. This domain makes specific hydrogen bonding interactions with all three bases of the GCA anticodon. The tRNA anticodon stem is bent sharply toward the enzyme as compared with its conformation when bound to elongation factor Tu, providing an essential basis for shape-selective recognition. The CysRS structure also reveals interactions of conserved enzyme groups with the sugar-phosphate backbone in the D loop, adjacent to an unusual G15.G48 tertiary base pair previously implicated in tRNA aminoacylation. A combined mutational analysis of enzyme and tRNA groups at G15.G48 supports the notion that contacts between CysRS and the sugar-phosphate backbone contribute to recognition by indirect readout.