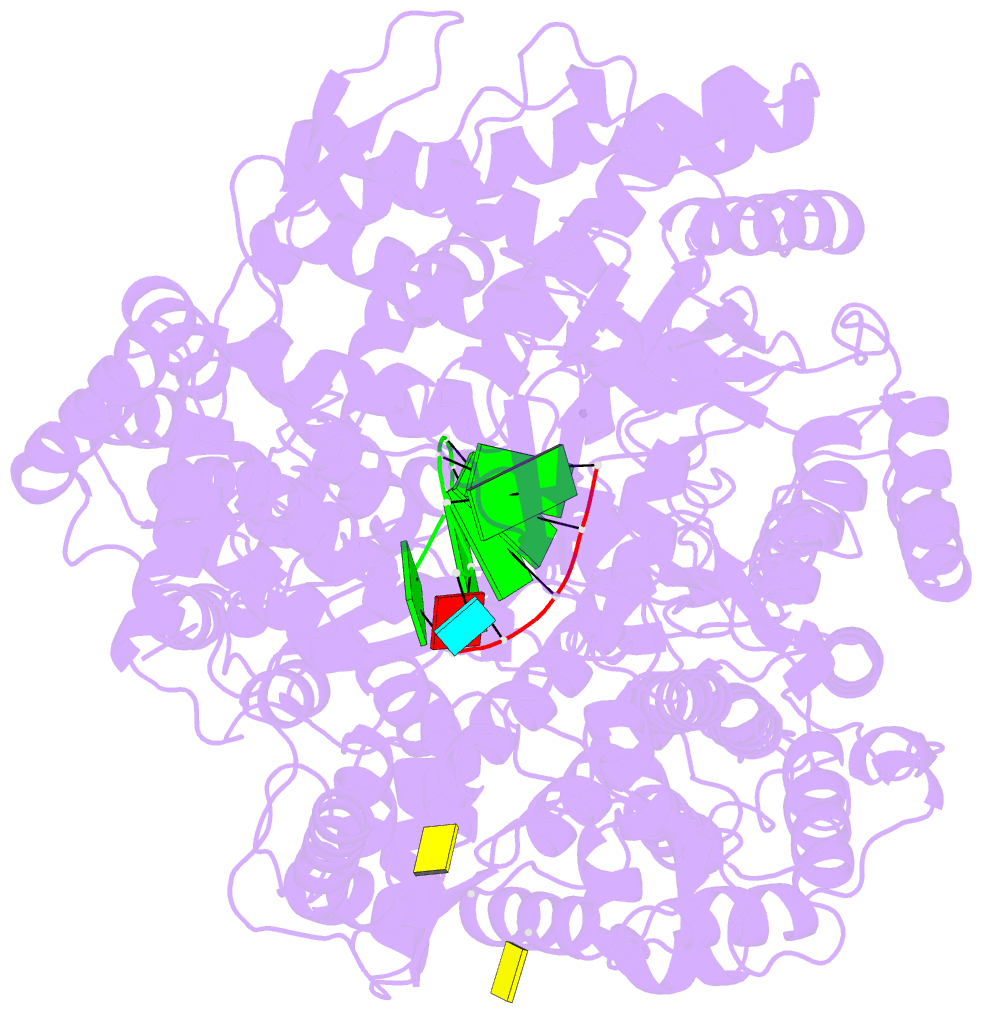
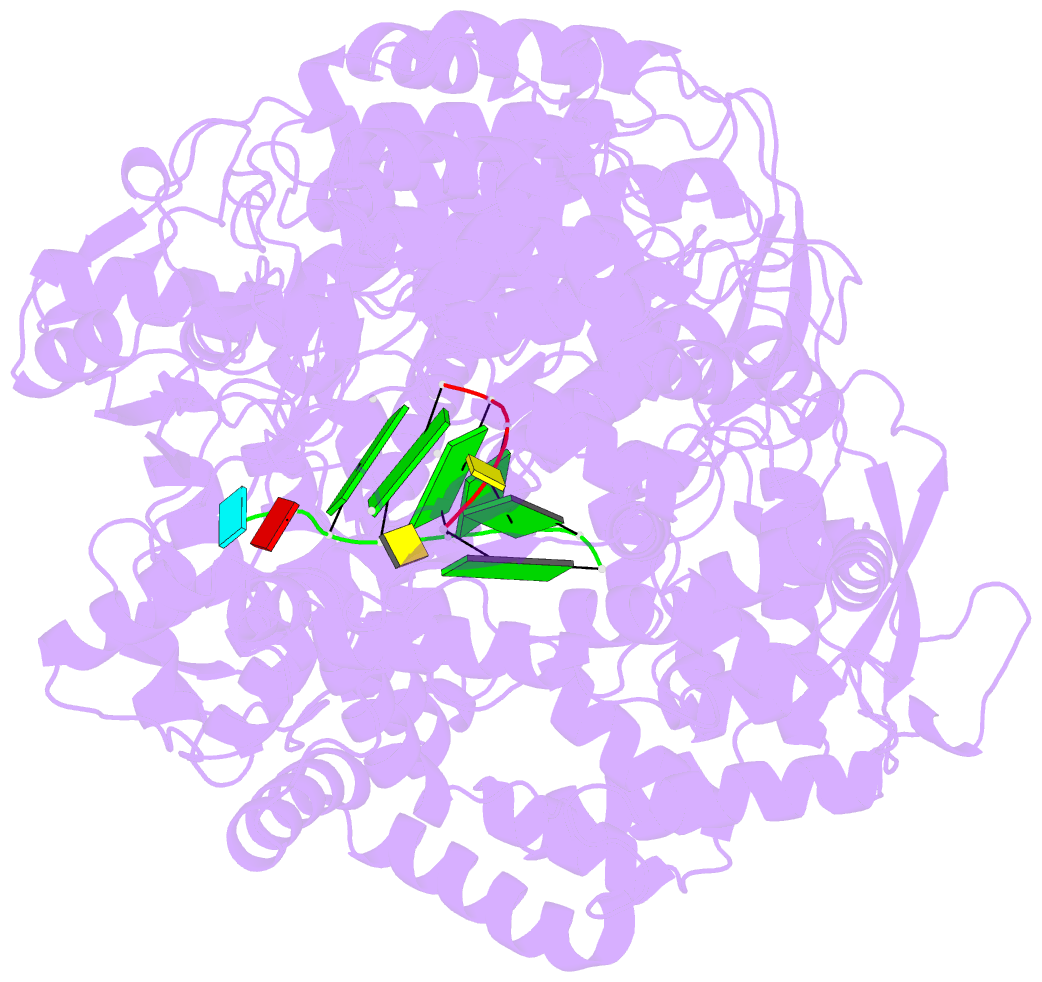
Summary information and primary citation
- PDB-id
- 1uon; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- polymerase
- Method
- cryo-EM (7.6 Å)
- Summary
- Reovirus polymerase lambda-3 localized by electron cryomicroscopy of virions at 7.6-a resolution
- Reference
- Zhang X, Walker SB, Chipman PR, Nibert ML, Baker TS (2003): "Reovirus Polymerase Lambda3 Localized by Cryo-Electron Microscopy of Virions at a Resolution of 7.6 A." Nat.Struct.Biol., 10, 1011. doi: 10.1038/NSB1009.
- Abstract
- Reovirus is an icosahedral, double-stranded (ds) RNA virus that uses viral polymerases packaged within the viral core to transcribe its ten distinct plus-strand RNAs. To localize these polymerases, the structure of the reovirion was refined to a resolution of 7.6 A by cryo-electron microscopy (cryo-EM) and three-dimensional (3D) image reconstruction. X-ray crystal models of reovirus proteins, including polymerase lambda 3, were then fitted into the density map. Each copy of lambda 3 was found anchored to the inner surface of the icosahedral core shell, making major contacts with three molecules of shell protein lambda 1 and overlapping, but not centering on, a five-fold axis. The overlap explains why only one copy of lambda 3 is bound per vertex. lambda 3 is furthermore oriented with its transcript exit channel facing a small channel through the lambda 1 shell, suggesting how the nascent RNA is passed into the large external cavity of the pentameric capping enzyme complex formed by protein lambda 2.