Summary information and primary citation
- PDB-id
- 1vas; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.75 Å)
- Summary
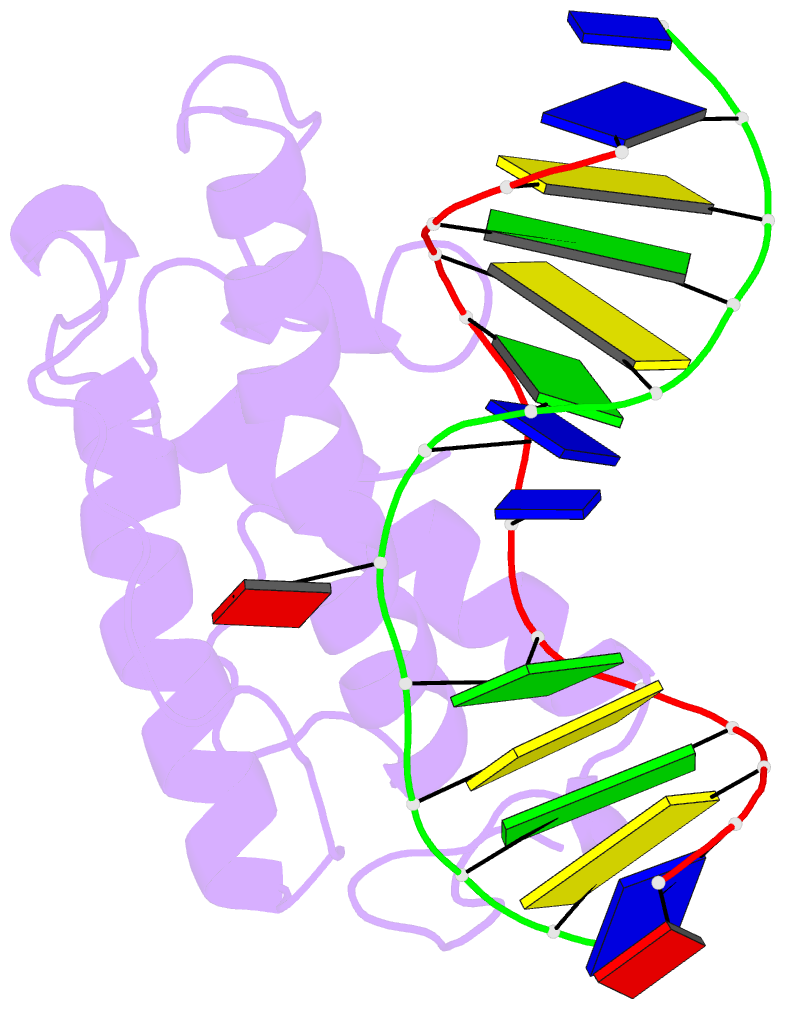
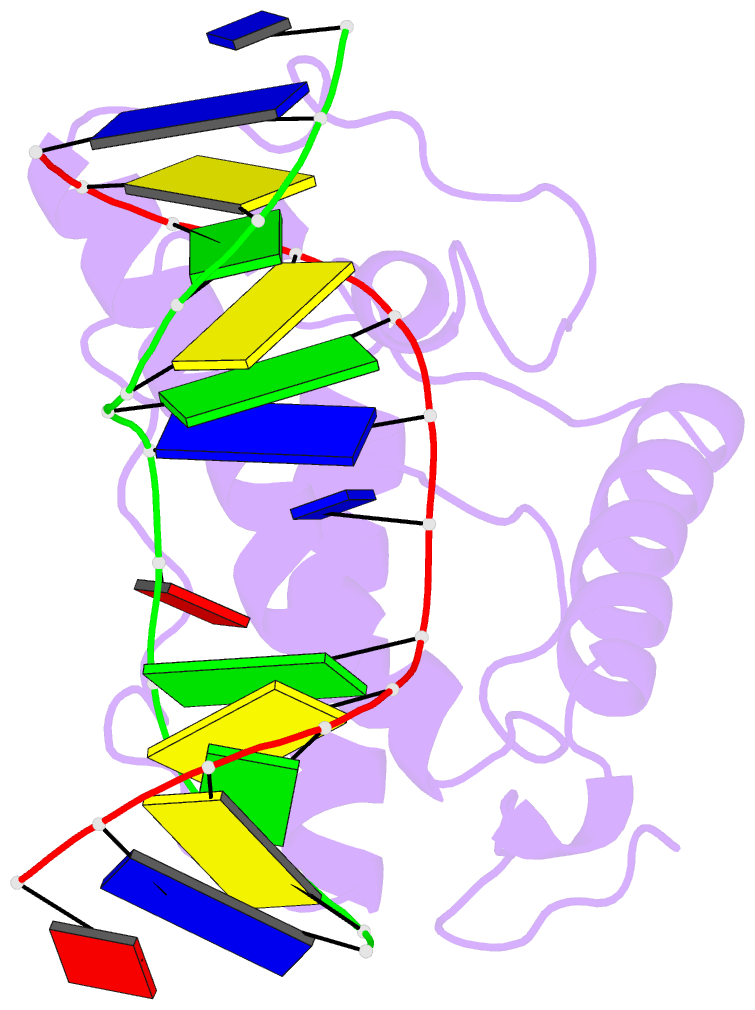
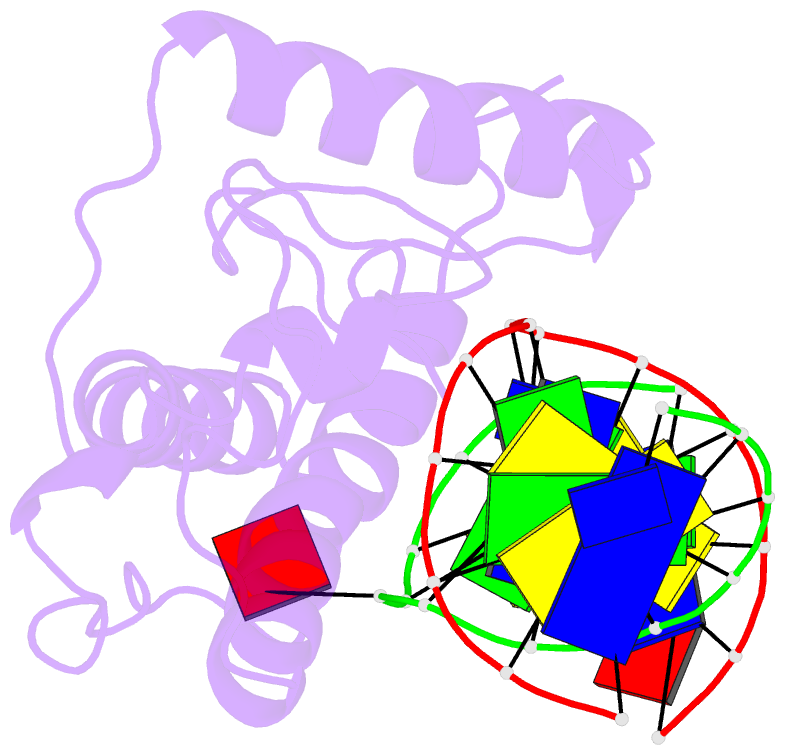
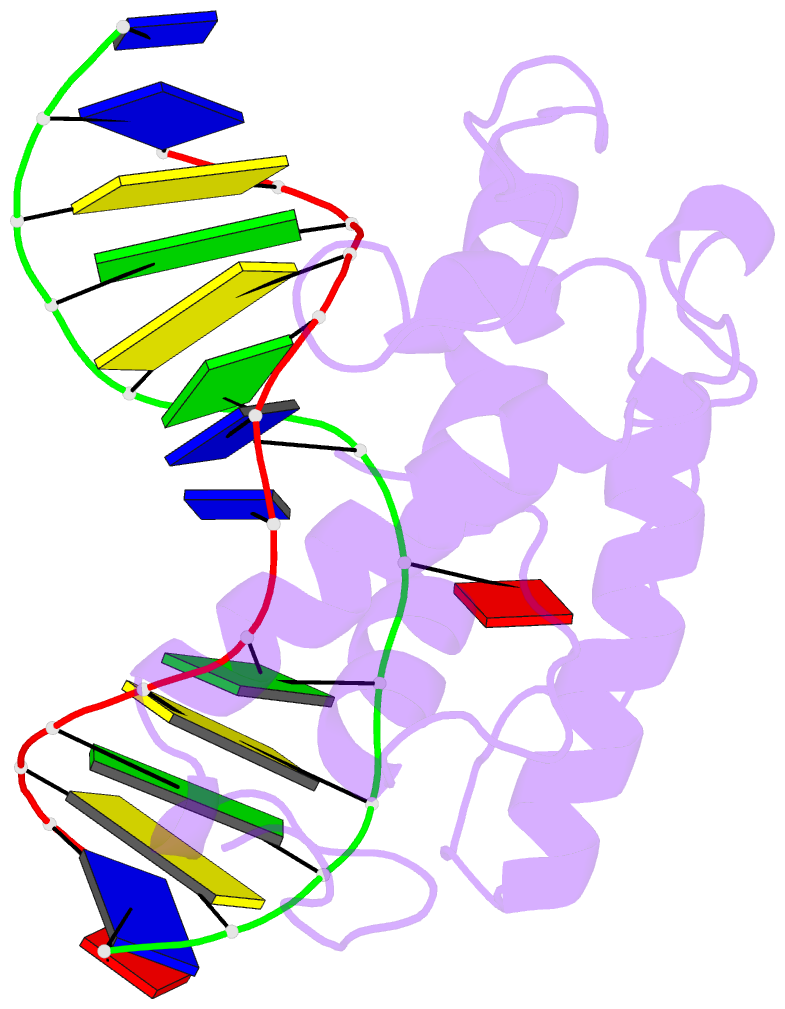
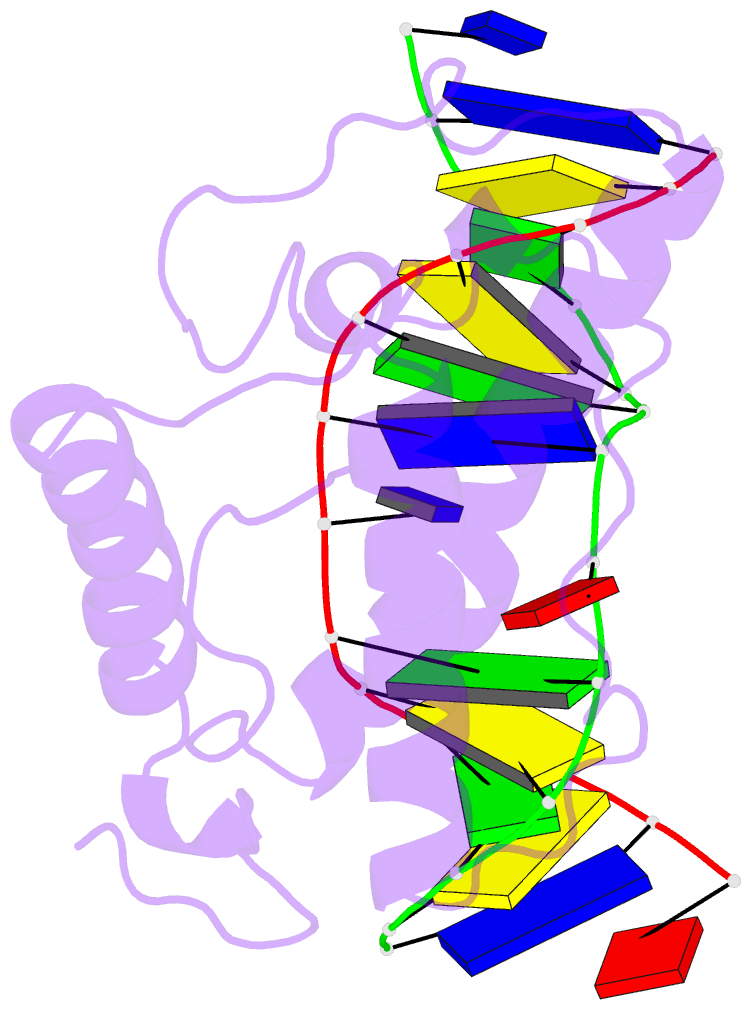
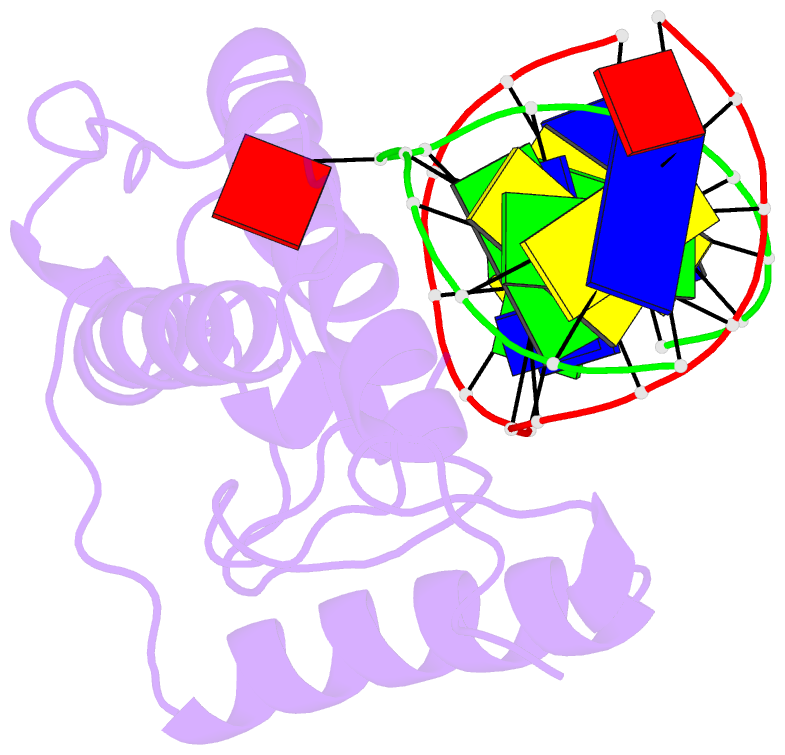
- Atomic model of a pyrimidine dimer specific excision repair enzyme complexed with a DNA substrate: structural basis for damaged DNA recognition
- Reference
- Vassylyev DG, Kashiwagi T, Mikami Y, Ariyoshi M, Iwai S, Ohtsuka E, Morikawa K (1995): "Atomic model of a pyrimidine dimer excision repair enzyme complexed with a DNA substrate: structural basis for damaged DNA recognition." Cell(Cambridge,Mass.), 83, 773-782. doi: 10.1016/0092-8674(95)90190-6.
- Abstract
- T4 endonuclease V is a DNA repair enzyme from bacteriophage T4 that catalyzes the first reaction step of the pyrimidine dimer-specific base excision repair pathway. The crystal structure of this enzyme complexed with a duplex DNA substrate, containing a thymine dimer, has been determined at 2.75 A resolution. The atomic structure of the complex reveals the unique conformation of the DNA duplex, which exhibits a sharp kink with a 60 degree inclination at the central thymine dimer. The adenine base complementary to the 5' side of the thymine dimer is completely flipped out of the DNA duplex and trapped in a cavity on the protein surface. These structural features allow an understanding of the catalytic mechanism and implicate a general mechanism of how other repair enzymes recognize damaged DNA duplexes.