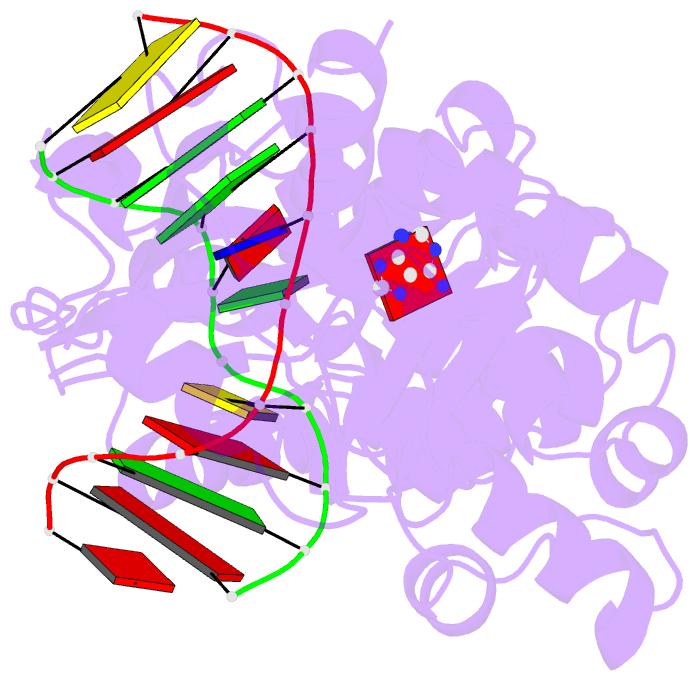
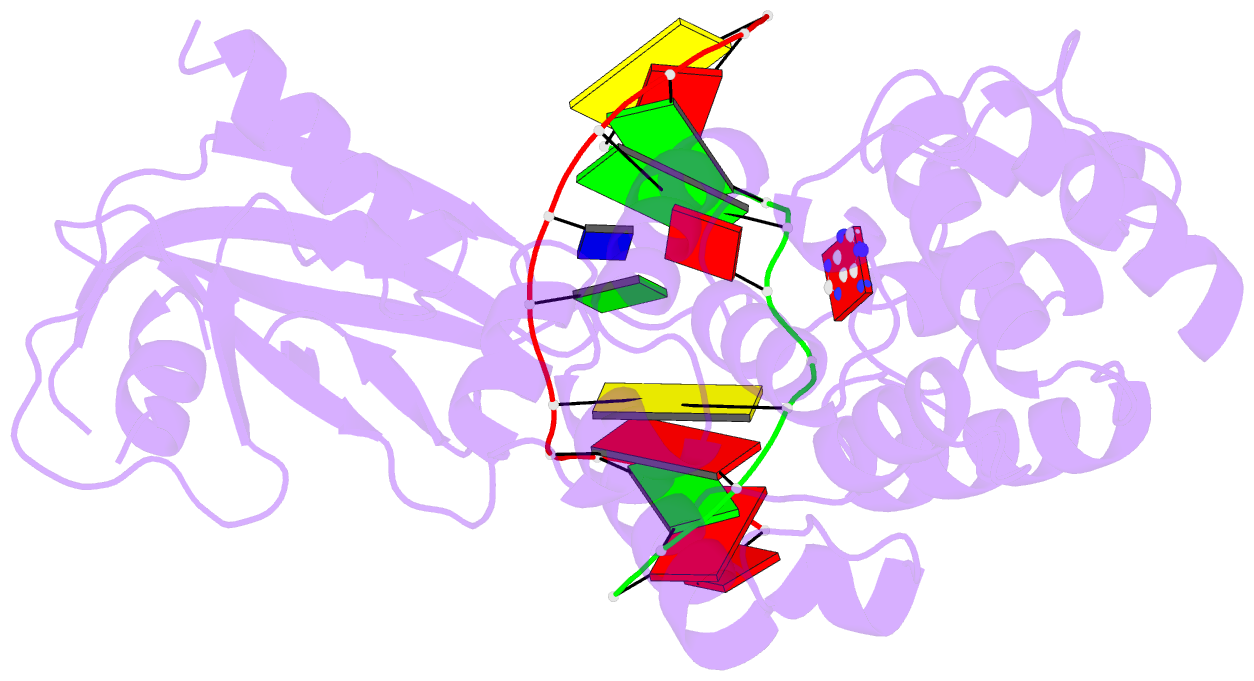
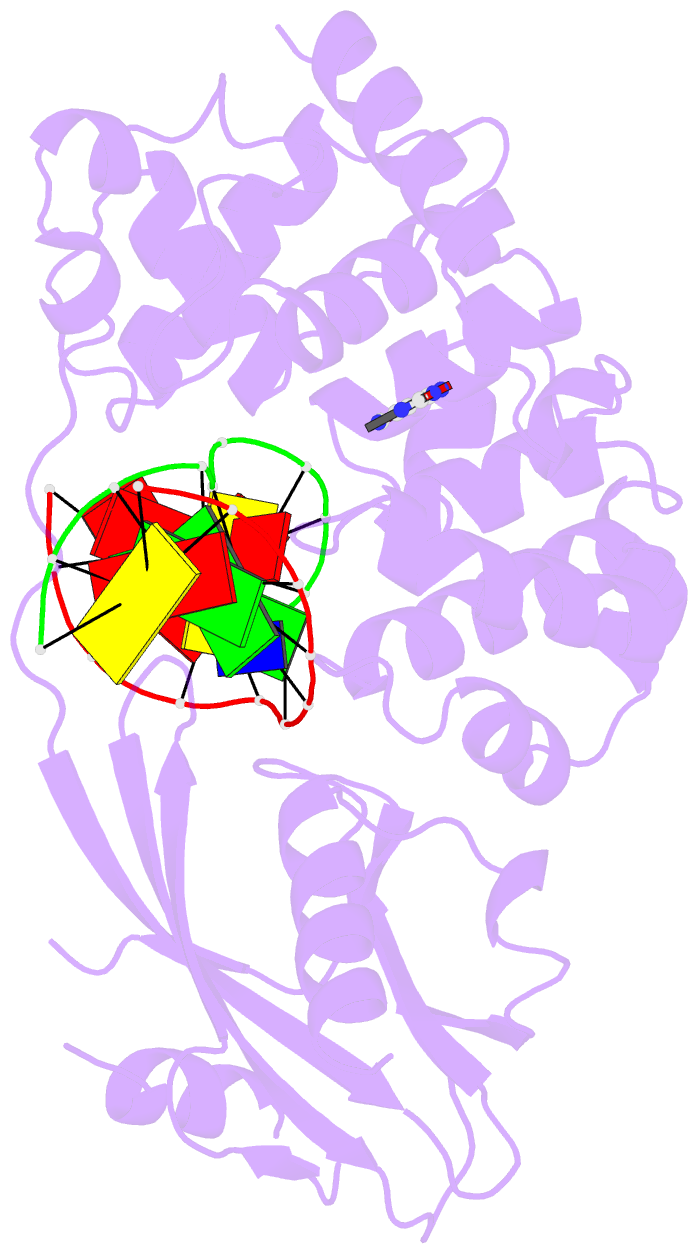
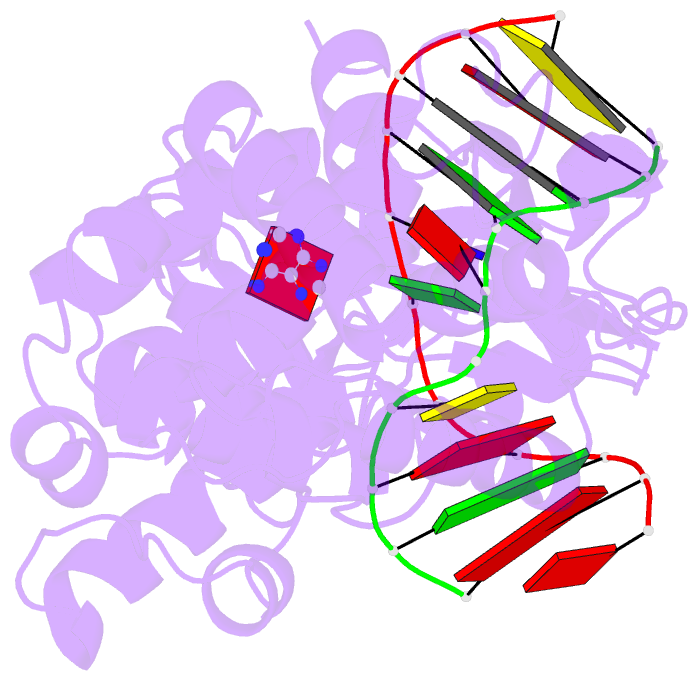
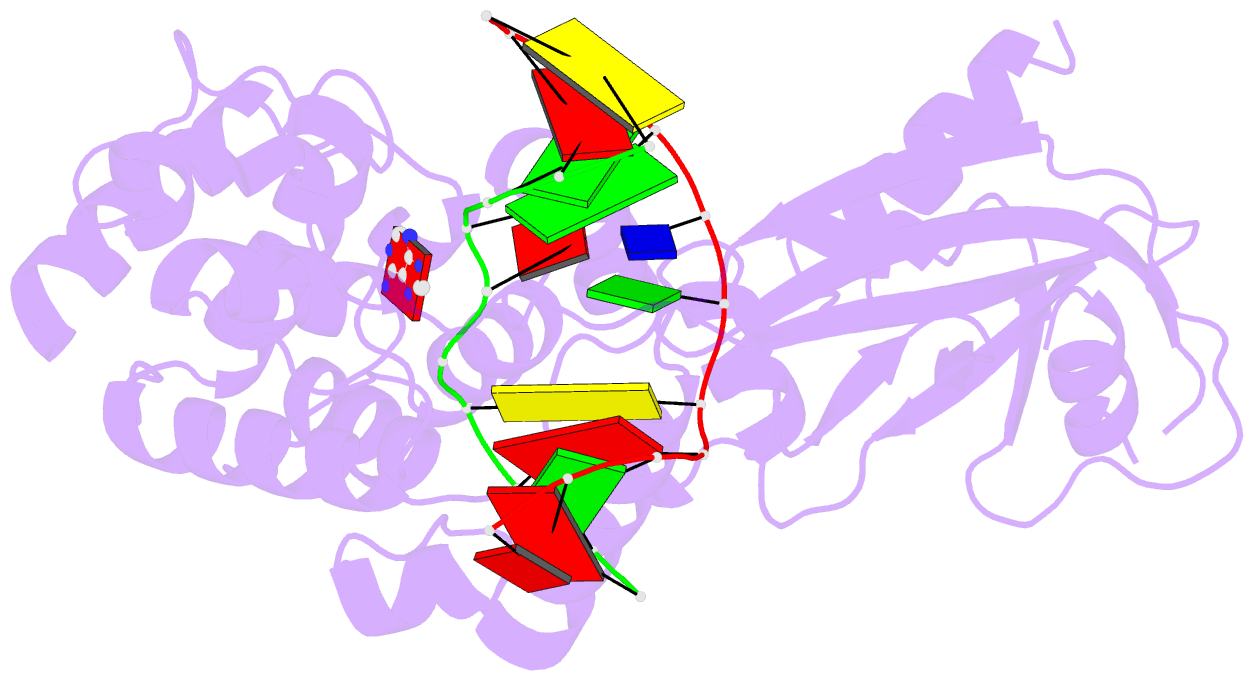
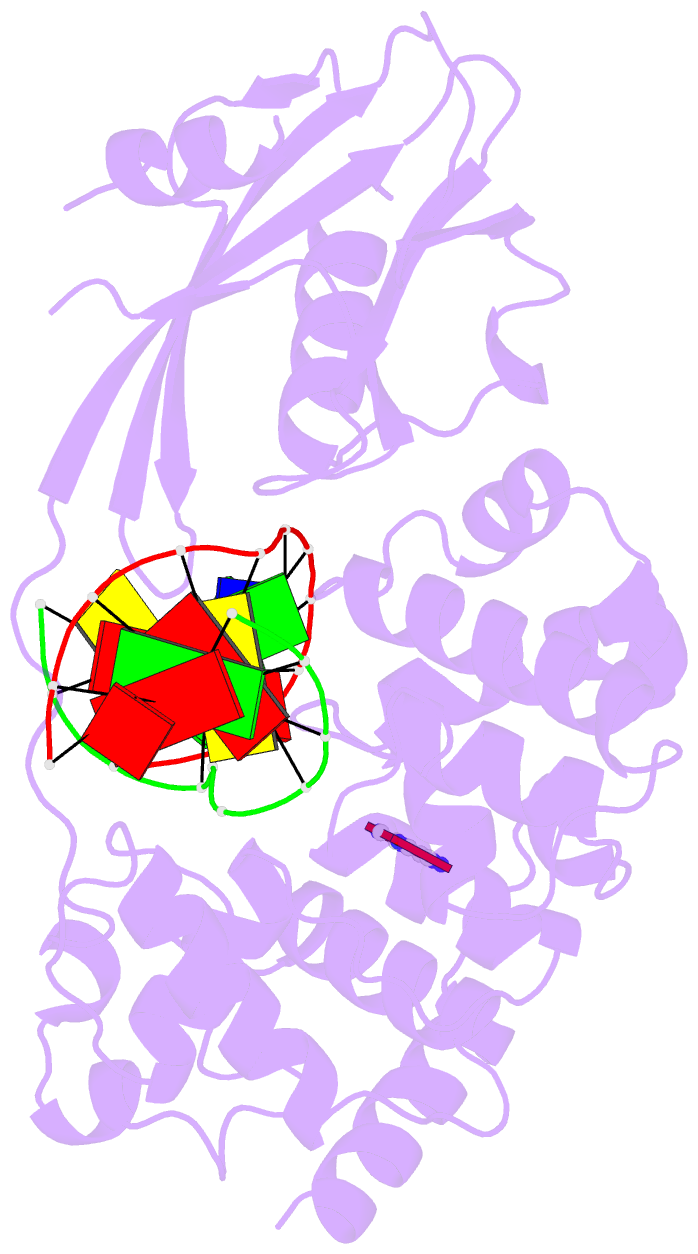
Summary information and primary citation
- PDB-id
- 1vrl; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.5 Å)
- Summary
- Muty adenine glycosylase in complex with DNA and soaked adenine free base
- Reference
- Fromme JC, Banerjee A, Huang SJ, Verdine GL (2004): "Structural basis for removal of adenine mispaired with 8-oxoguanine by MutY adenine DNA glycosylase." Nature, 427, 652-656. doi: 10.1038/nature02306.
- Abstract
- The genomes of aerobic organisms suffer chronic oxidation of guanine to the genotoxic product 8-oxoguanine (oxoG). Replicative DNA polymerases misread oxoG residues and insert adenine instead of cytosine opposite the oxidized base. Both bases in the resulting A*oxoG mispair are mutagenic lesions, and both must undergo base-specific replacement to restore the original C*G pair. Doing so represents a formidable challenge to the DNA repair machinery, because adenine makes up roughly 25% of the bases in most genomes. The evolutionarily conserved enzyme adenine DNA glycosylase (called MutY in bacteria and hMYH in humans) initiates repair of A*oxoG to C*G by removing the inappropriately paired adenine base from the DNA backbone. A central issue concerning MutY function is the mechanism by which A*oxoG mispairs are targeted among the vast excess of A*T pairs. Here we report the use of disulphide crosslinking to obtain high-resolution crystal structures of MutY-DNA lesion-recognition complexes. These structures reveal the basis for recognizing both lesions in the A*oxoG pair and for catalysing removal of the adenine base.