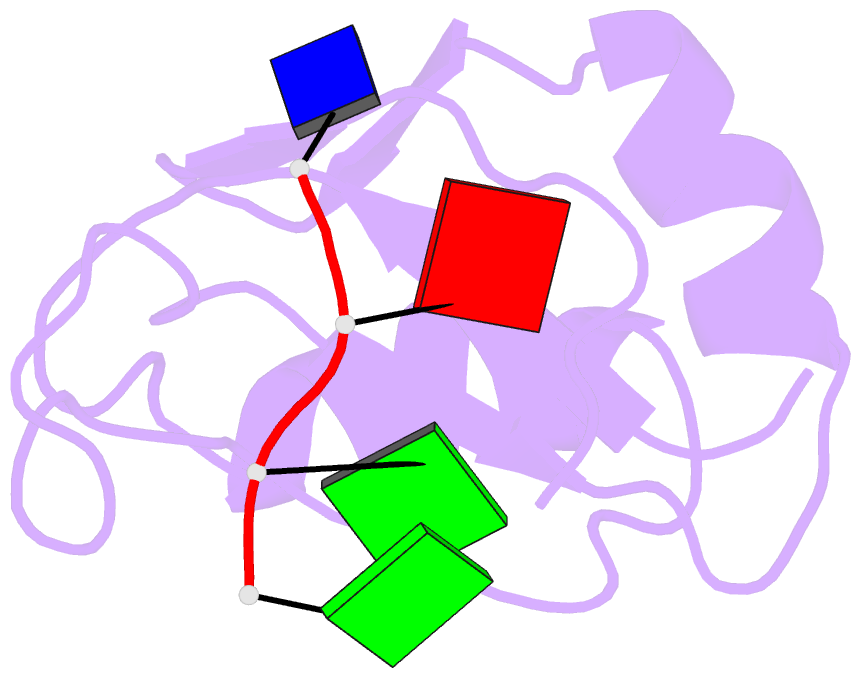
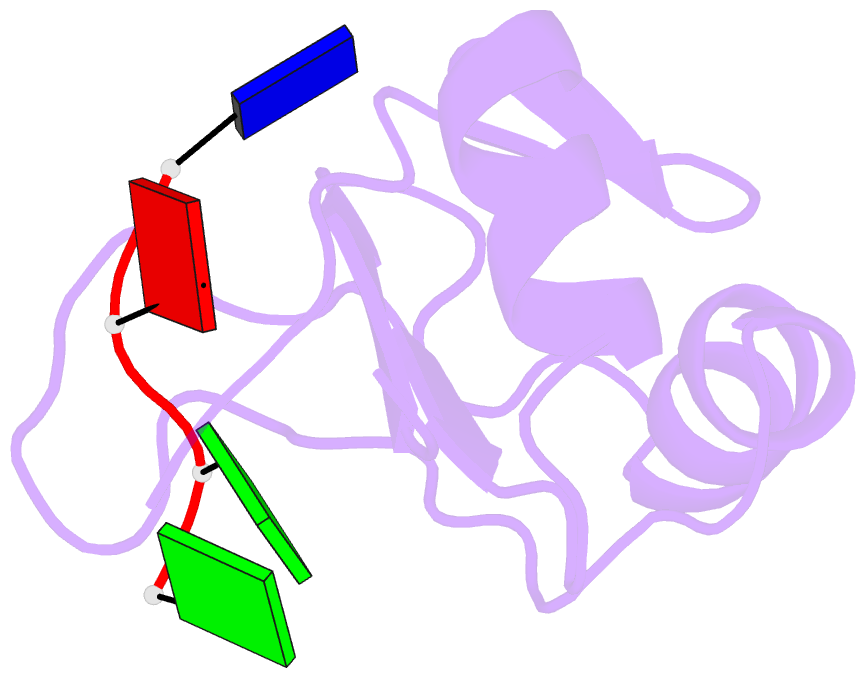
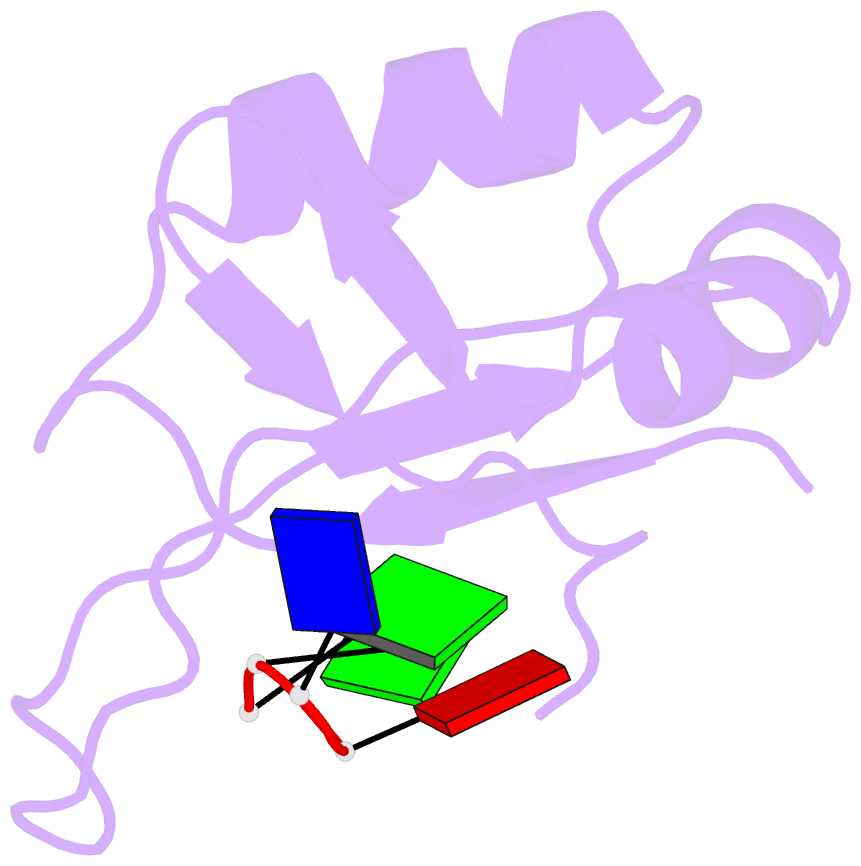
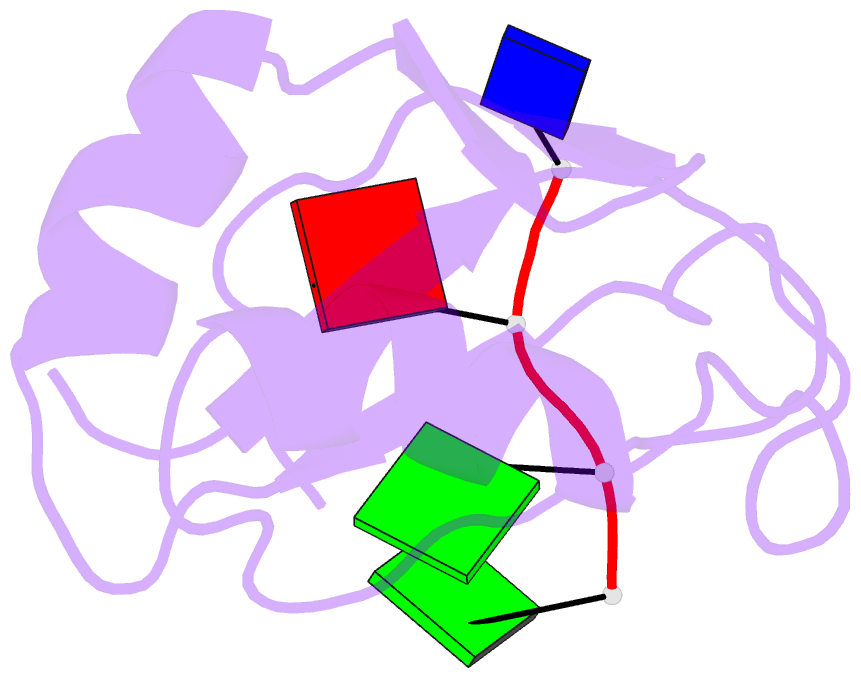
Summary information and primary citation
- PDB-id
- 1wtb; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- NMR
- Summary
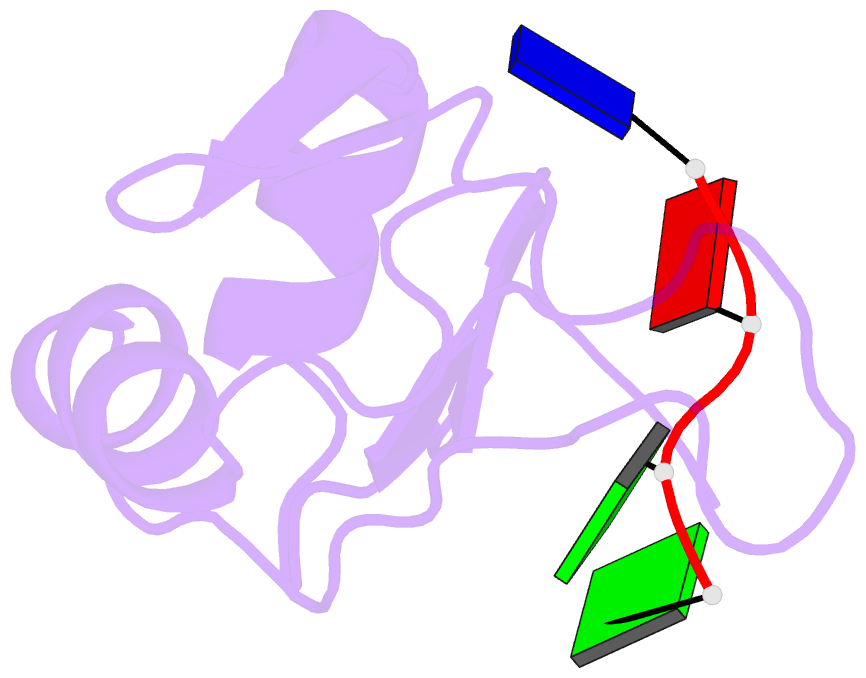
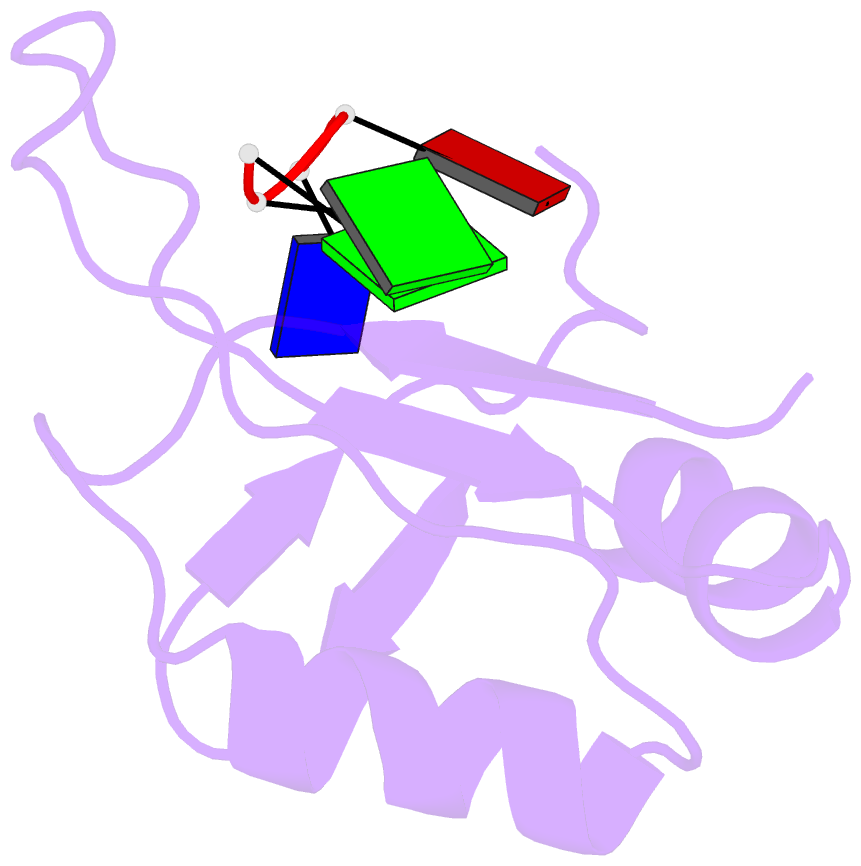
- Complex structure of the c-terminal RNA-binding domain of hnrnp d (auf1) with telomere DNA
- Reference
- Enokizono Y, Konishi Y, Nagata K, Ouhashi K, Uesugi S, Ishikawa F, Katahira M (2005): "Structure of hnRNP D complexed with single-stranded telomere DNA and unfolding of the quadruplex by heterogeneous nuclear ribonucleoprotein D." J.Biol.Chem., 280, 18862-18870. doi: 10.1074/jbc.M411822200.
- Abstract
- Heterogeneous nuclear ribonucleoprotein D, also known as AUF1, has two DNA/RNA-binding domains, each of which can specifically bind to single-stranded d(TTAGGG)n, the human telomeric repeat. Here, the structure of the C-terminal-binding domain (BD2) complexed with single-stranded d(TTAGGG) determined by NMR is presented. The structure has revealed that each residue of the d(TAG) segment is recognized by BD2 in a base-specific manner. The interactions deduced from the structure have been confirmed by gel retardation experiments with mutant BD2 and DNA. It is known that single-stranded DNA with the telomeric repeat tends to form a quadruplex and that the quadruplex has an inhibitory effect on telomere elongation by telomerase. This time it is revealed that BD2 unfolds the quadruplex of such DNA upon binding. Moreover, the effect of BD2 on the elongation by telomerase was examined in vitro. These results suggest the possible involvement of heterogeneous nuclear ribonucleoprotein D in maintenance of the telomere 3'-overhang either through protection of a single-stranded DNA or destabilization of the potentially deleterious quadruplex structure for the elongation by telomerase.