Summary information and primary citation
- PDB-id
- 1xbr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.5 Å)
- Summary
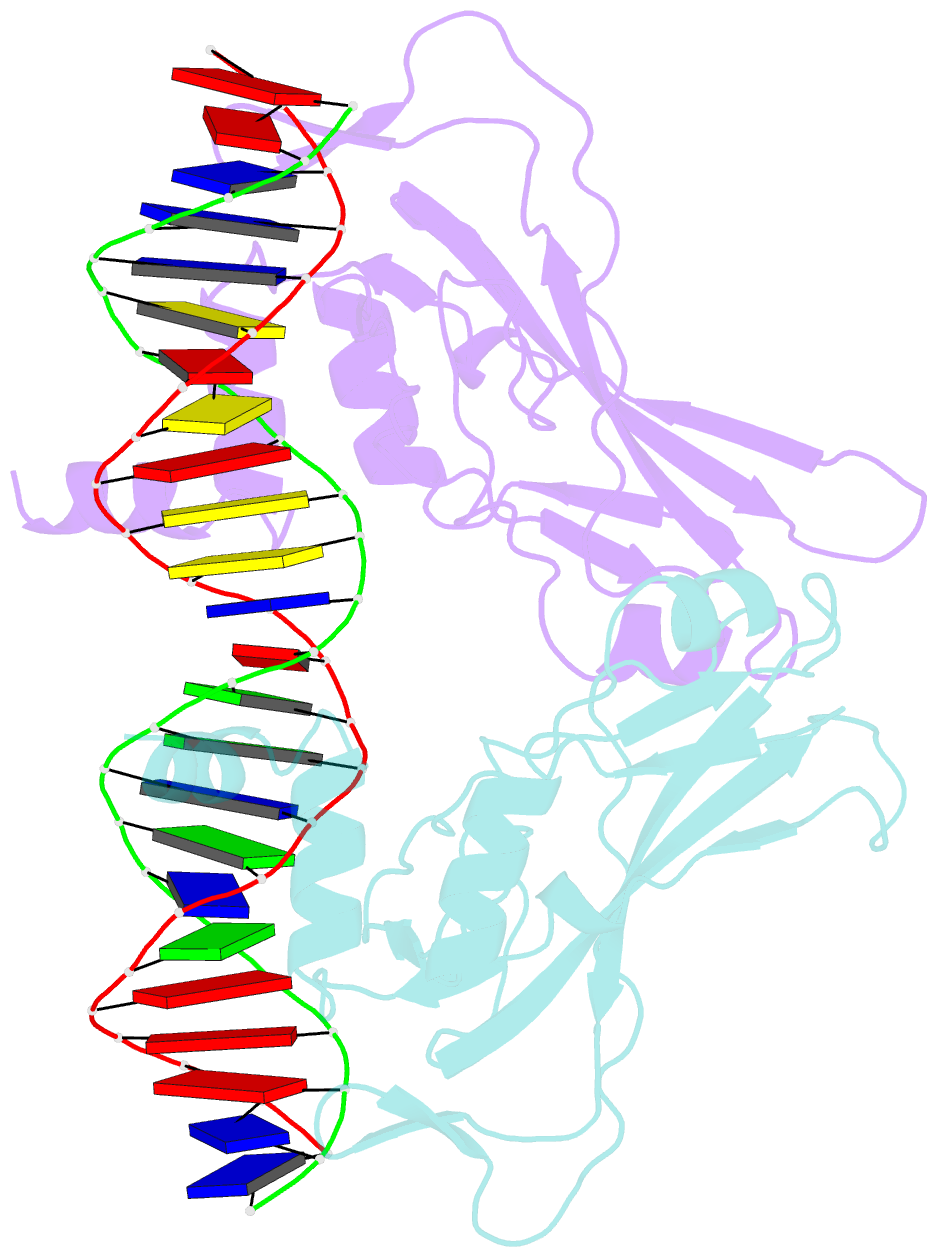
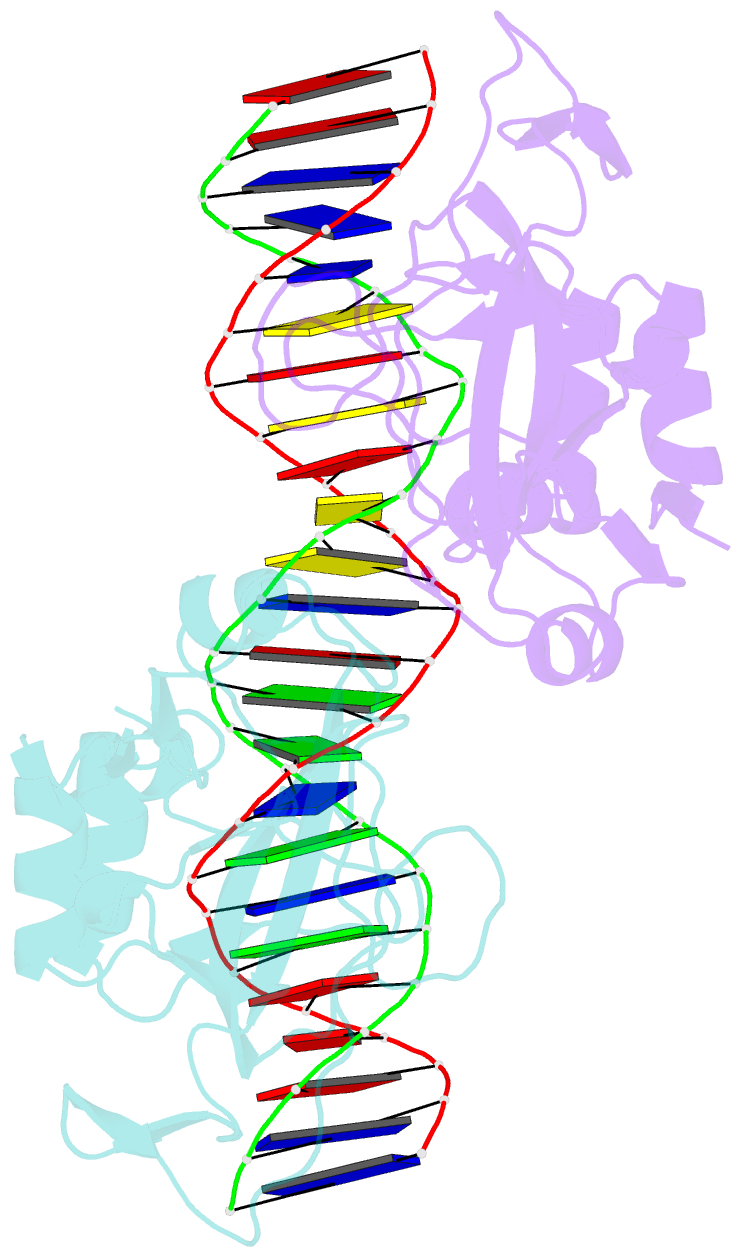
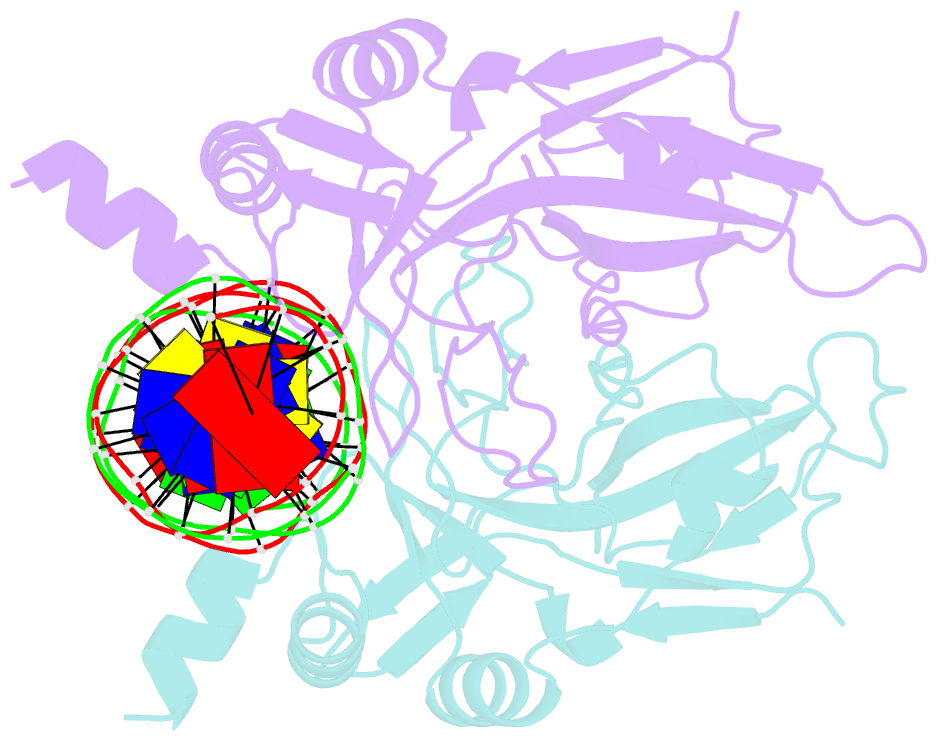
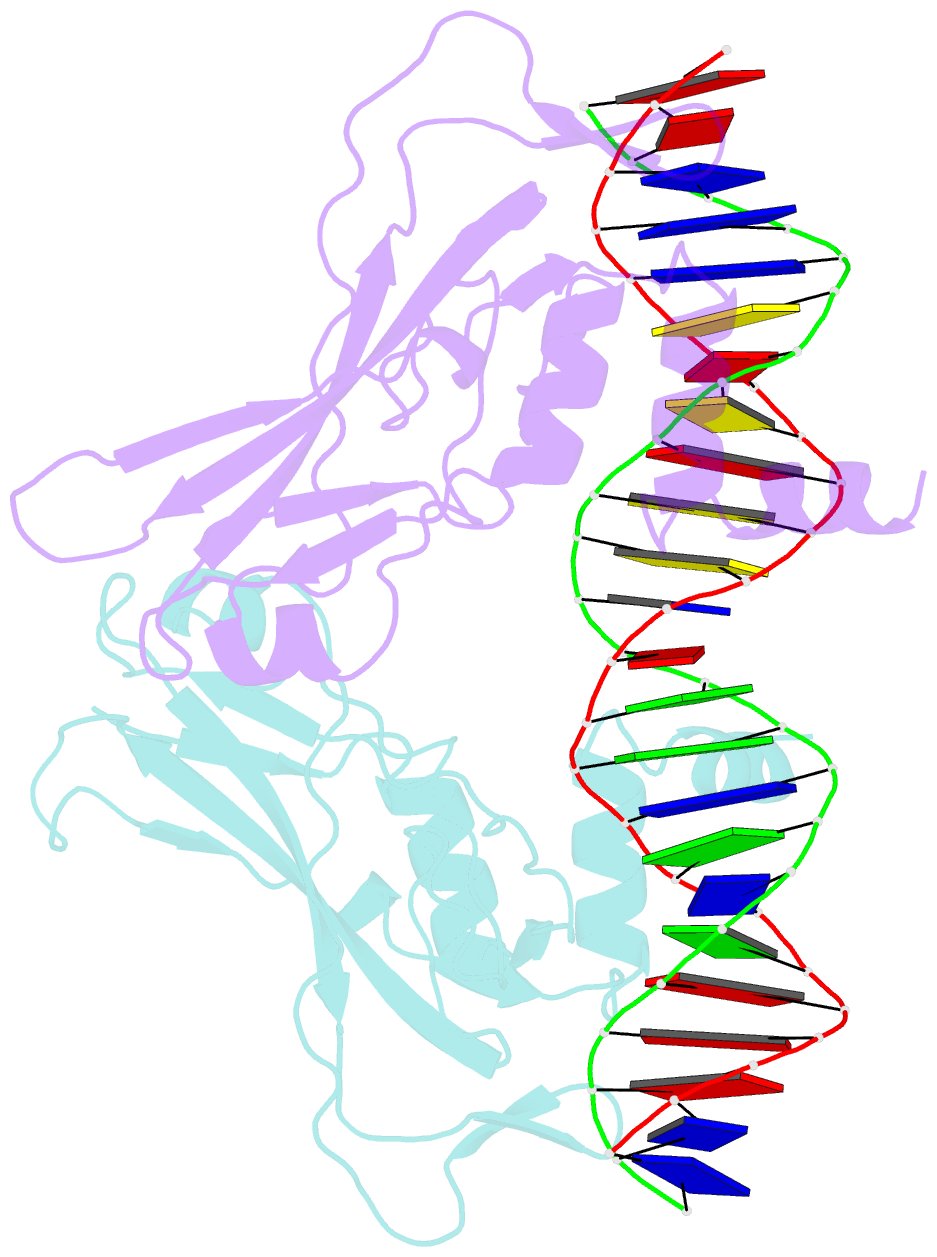
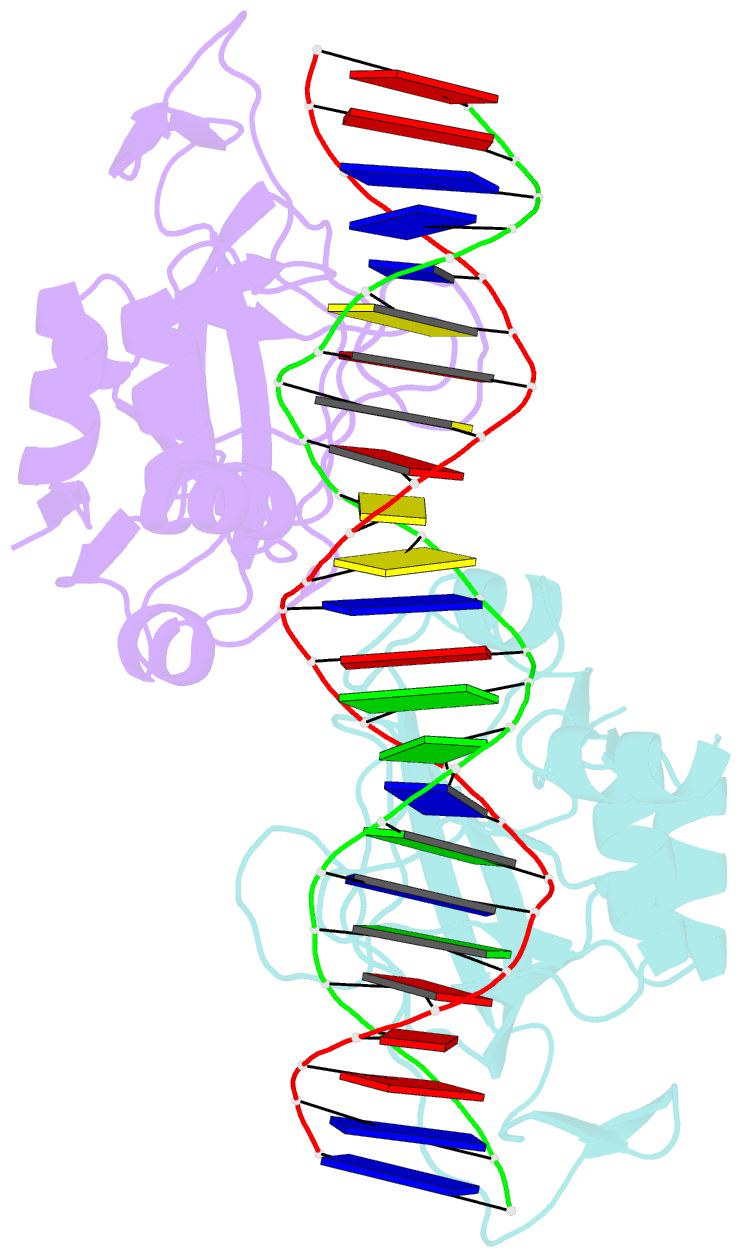
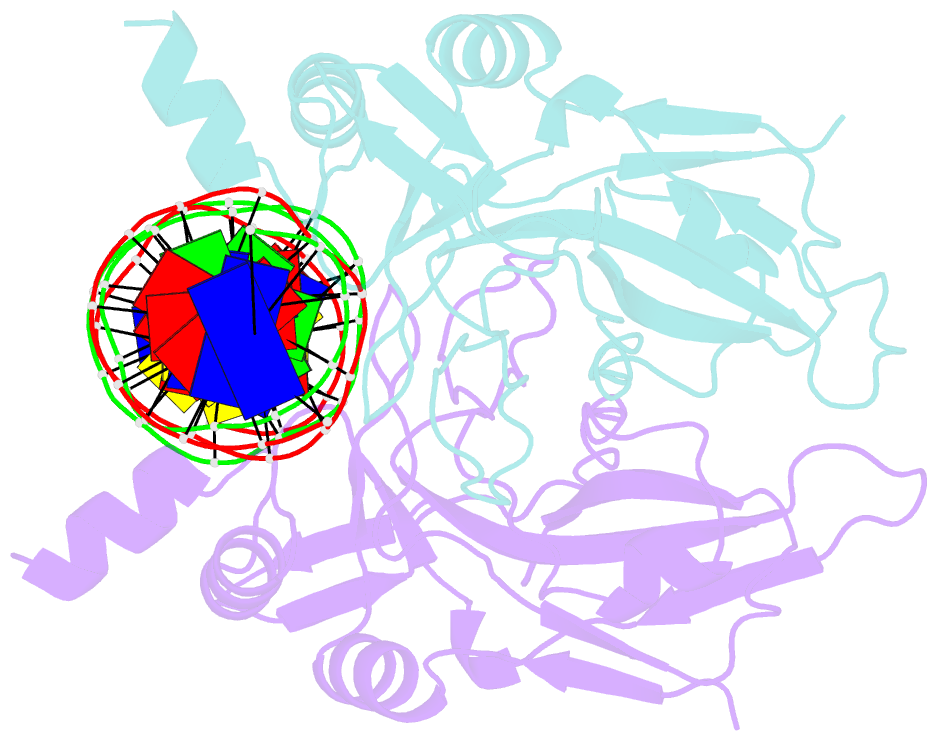
- T domain from xenopus laevis bound to DNA
- Reference
- Muller CW, Herrmann BG (1997): "Crystallographic structure of the T domain-DNA complex of the Brachyury transcription factor." Nature, 389, 884-888. doi: 10.1038/39929.
- Abstract
- The mouse Brachyury (T) gene is the prototype of a growing family of so-called T-box genes which encode transcriptional regulators and have been identified in a variety of invertebrates and vertebrates, including humans. Mutations in Brachyury and other T-box genes result in drastic embryonic phenotypes, indicating that T-box gene products are essential in tissue specification, morphogenesis and organogenesis. The T-box encodes a DNA-binding domain of about 180 amino-acid residues, the T domain. Here we report the X-ray structure of the T domain from Xenopus laevis in complex with a 24-nucleotide palindromic DNA duplex. We show that the protein is bound as a dimer, interacting with the major and the minor grooves of the DNA. A new type of specific DNA contact is seen, in which a carboxy-terminal helix is deeply embedded into an enlarged minor groove without bending the DNA. Hydrophobic interactions and an unusual main-chain carbonyl contact to a guanine account for sequence-specific recognition in the minor groove by this helix. Thus the structure of this T domain complex with DNA reveals a new way in which a protein can recognize DNA.