Summary information and primary citation
- PDB-id
- 1xjv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (1.73 Å)
- Summary
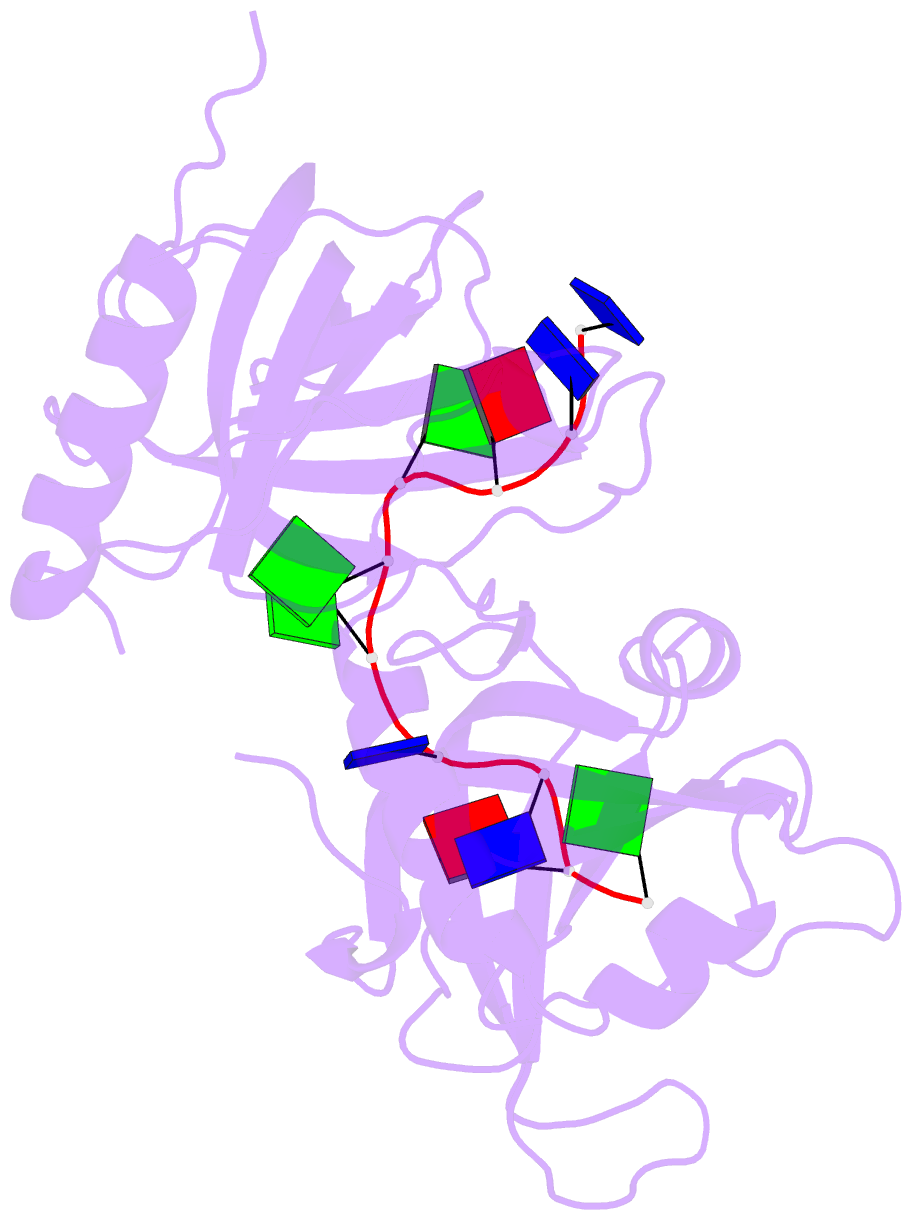
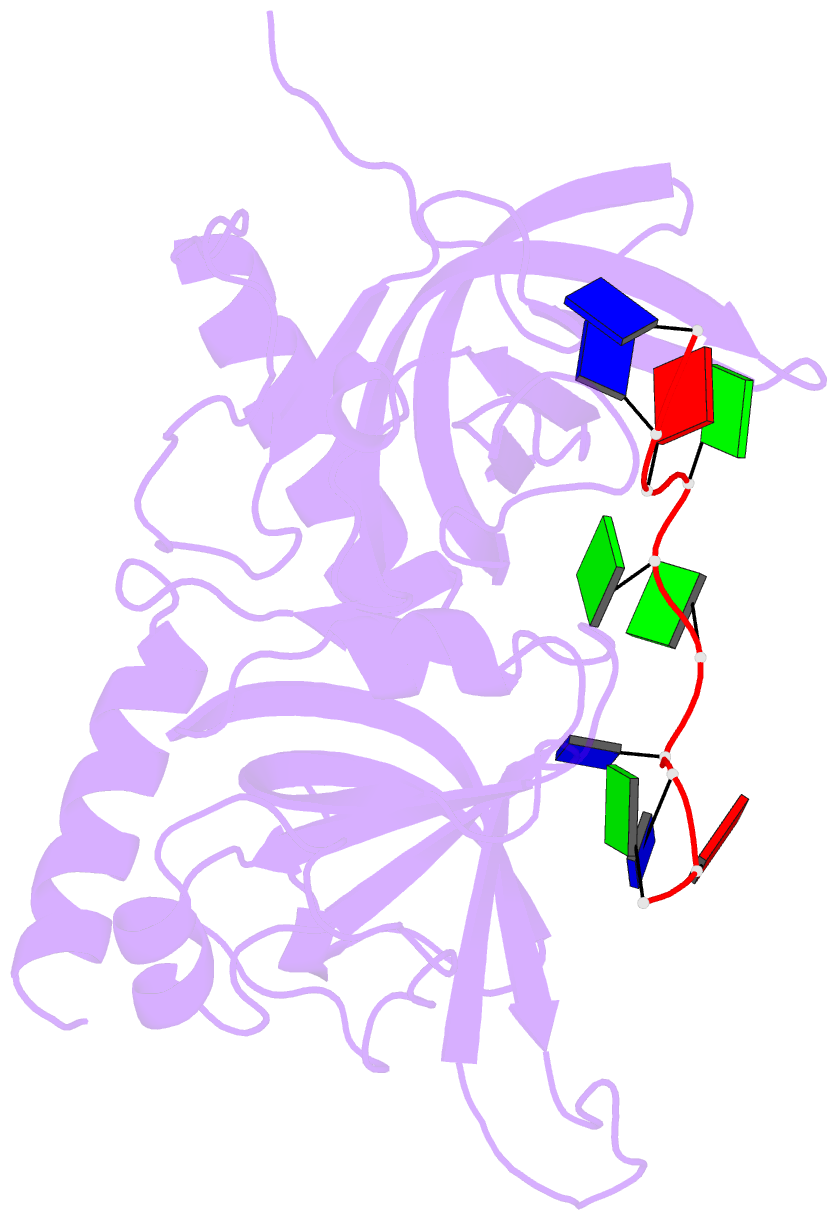
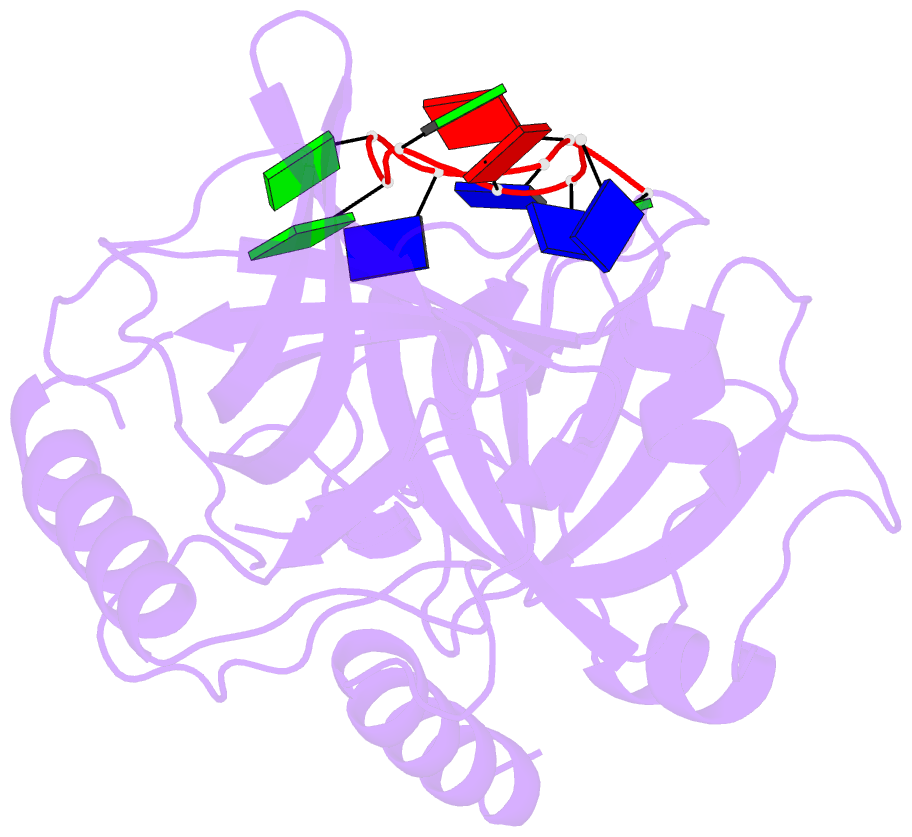
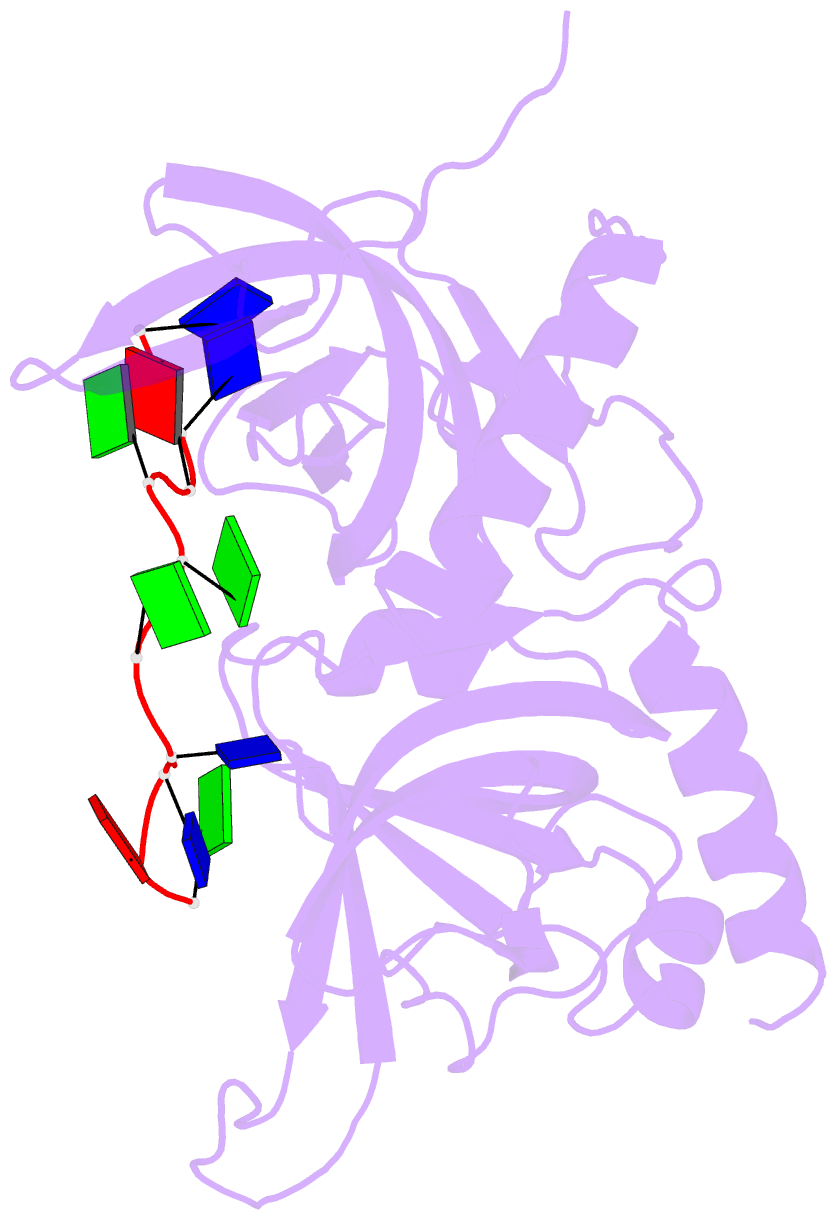
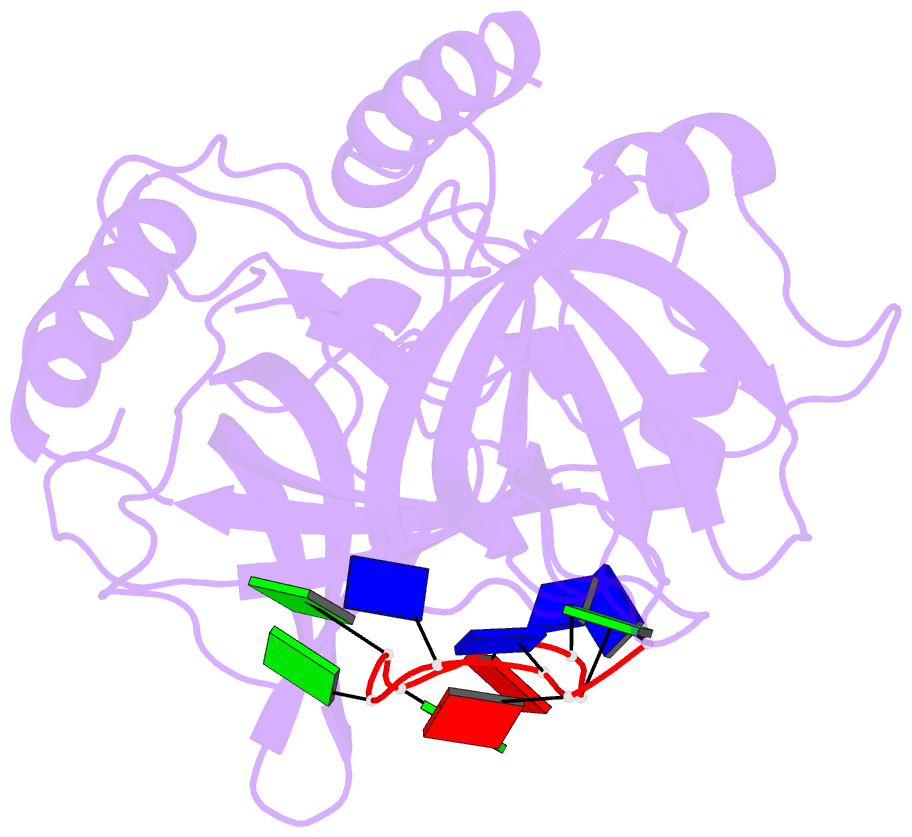
- Crystal structure of human pot1 bound to telomeric single-stranded DNA (ttagggttag)
- Reference
- Lei M, Podell ER, Cech TR (2004): "Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection." Nat.Struct.Mol.Biol., 11, 1223-1229. doi: 10.1038/nsmb867.
- Abstract
- The POT1 (protection of telomeres 1) protein binds the single-stranded overhang at the ends of chromosomes in diverse eukaryotes. It is essential for chromosome end-protection in the fission yeast Schizosaccharomyces pombe, and it is involved in regulation of telomere length in human cells. Here, we report the crystal structure at a resolution of 1.73 A of the N-terminal half of human POT1 (hPOT1) protein bound to a telomeric single-stranded DNA (ssDNA) decamer, TTAGGGTTAG, the minimum tight-binding sequence indicated by in vitro binding assays. The structure reveals that hPOT1 contains two oligonucleotide/ oligosaccharide-binding (OB) folds; the N-terminal OB fold binds the first six nucleotides, resembling the structure of the S. pombe Pot1pN-ssDNA complex, whereas the second OB fold binds and protects the 3' end of the ssDNA. These results provide an atomic-resolution model for chromosome end-capping.