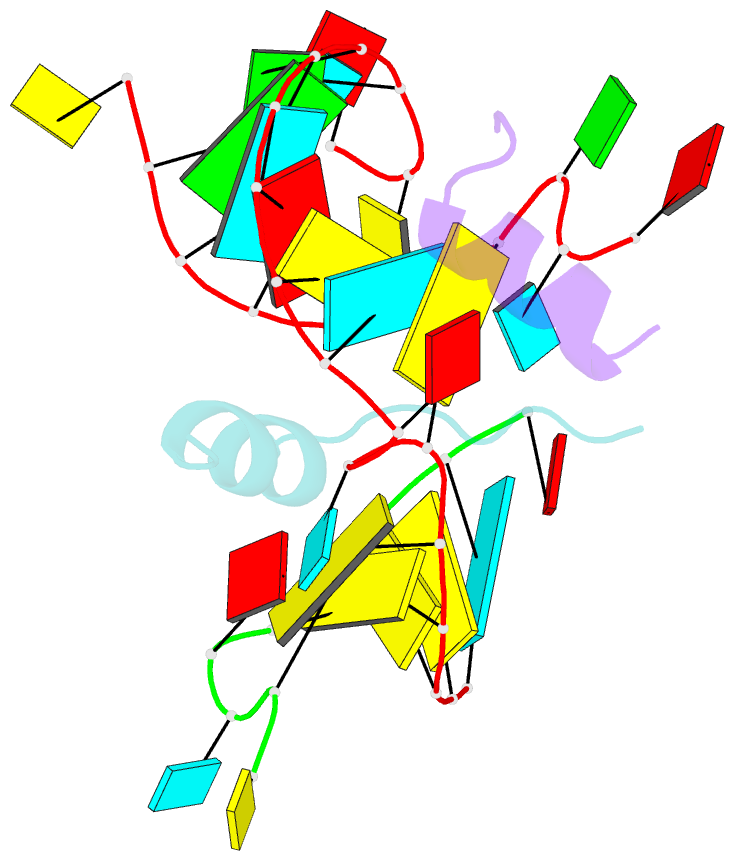
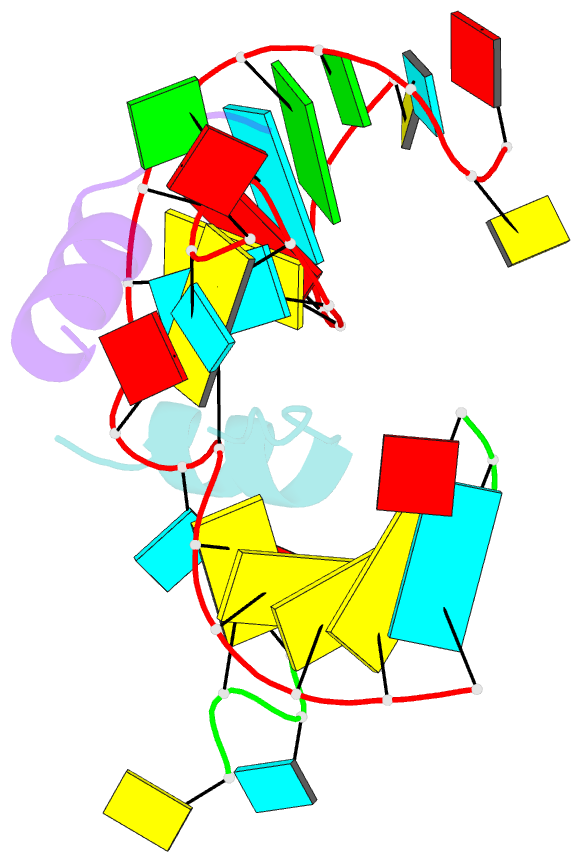
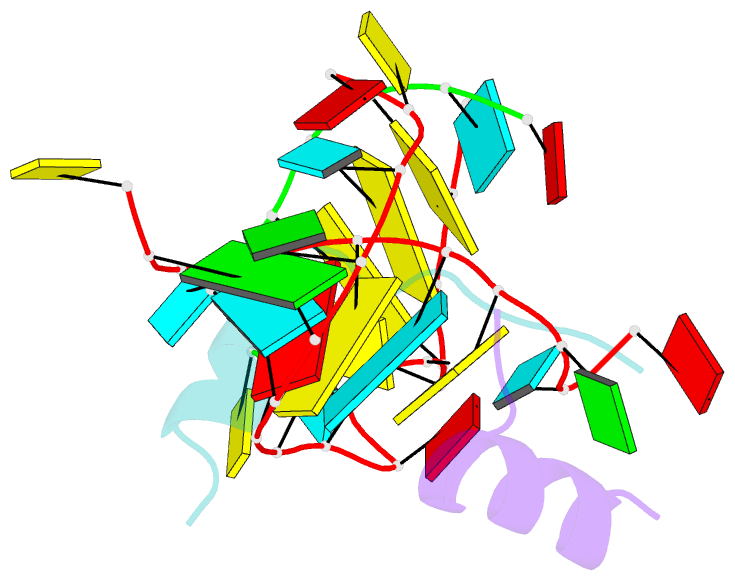
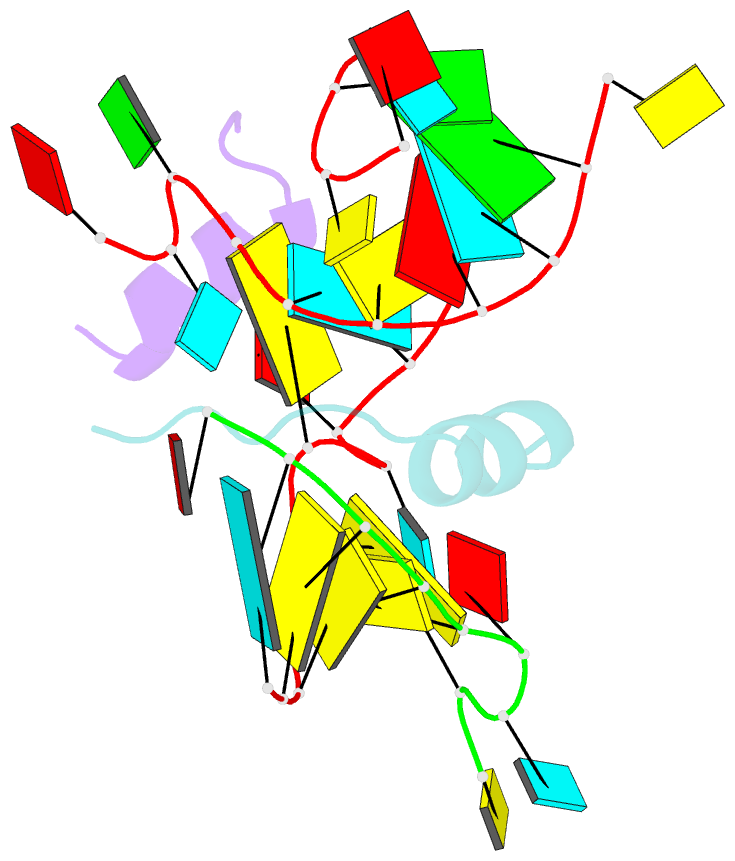
Summary information and primary citation
- PDB-id
- 1xok; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-RNA
- Method
- X-ray (3.0 Å)
- Summary
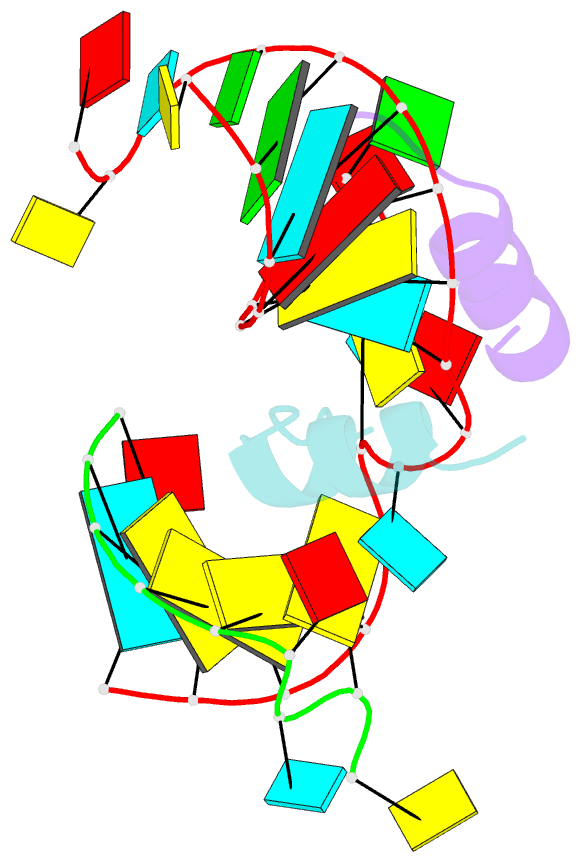
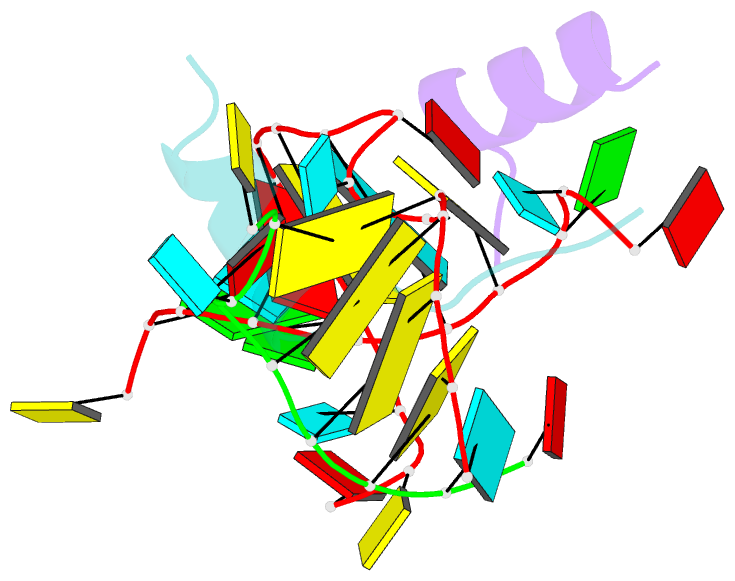
- Crystal structure of alfalfa mosaic virus RNA 3'utr in complex with coat protein n terminal peptide
- Reference
- Guogas LM, Filman DJ, Hogle JM, Gehrke L (2004): "Cofolding organizes alfalfa mosaic virus RNA and coat protein for replication." Science, 306, 2108-2111. doi: 10.1126/science.1103399.
- Abstract
- Alfalfa mosaic virus genomic RNAs are infectious only when the viral coat protein binds to the RNA 3' termini. The crystal structure of an alfalfa mosaic virus RNA-peptide complex reveals that conserved AUGC repeats and Pro-Thr-x-Arg-Ser-x-x-Tyr coat protein amino acids cofold upon interacting. Alternating AUGC residues have opposite orientation, and they base pair in different adjacent duplexes. Localized RNA backbone reversals stabilized by arginine-guanine interactions place the adenosines and guanines in reverse order in the duplex. The results suggest that a uniform, organized 3' conformation, similar to that found on viral RNAs with transfer RNA-like ends, may be essential for replication.