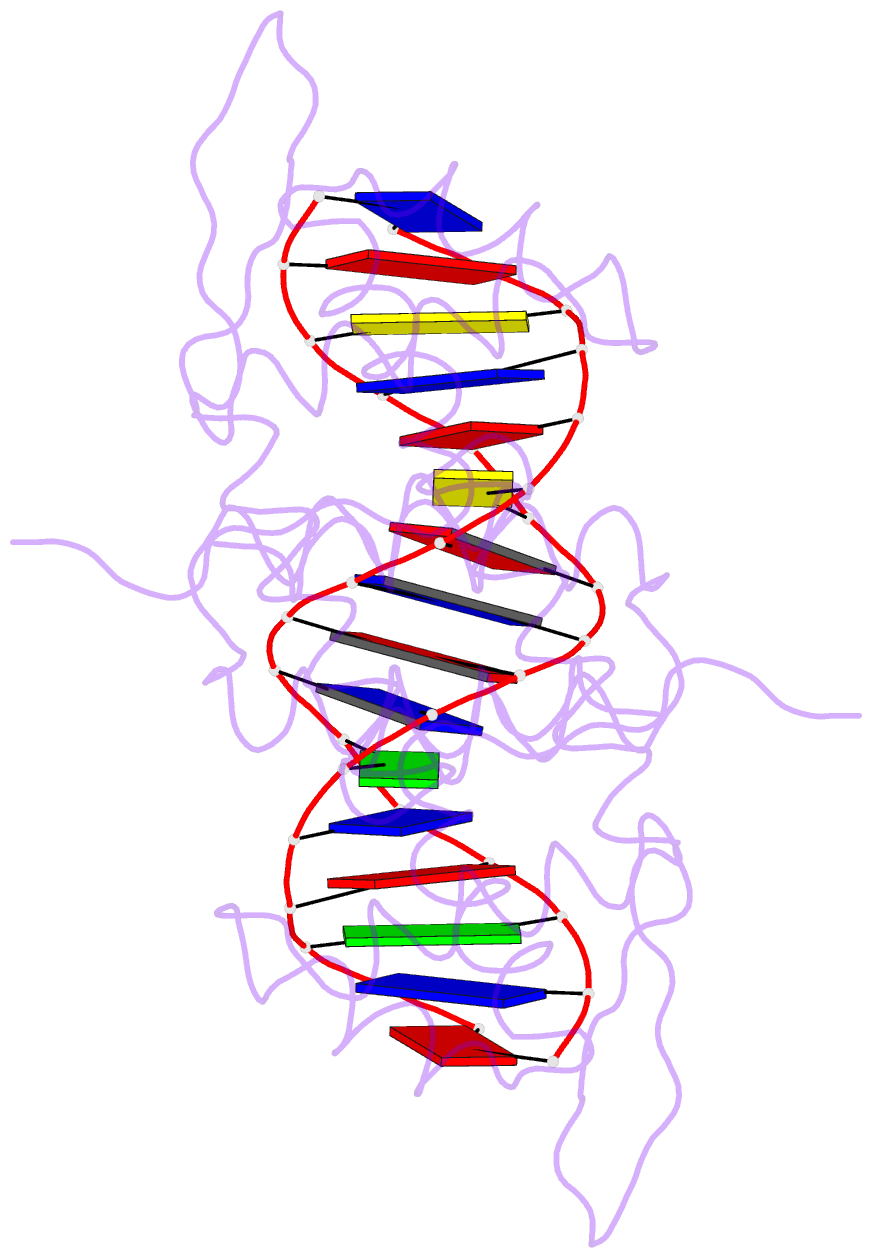
Summary information and primary citation
- PDB-id
- 1xsd; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.7 Å)
- Summary
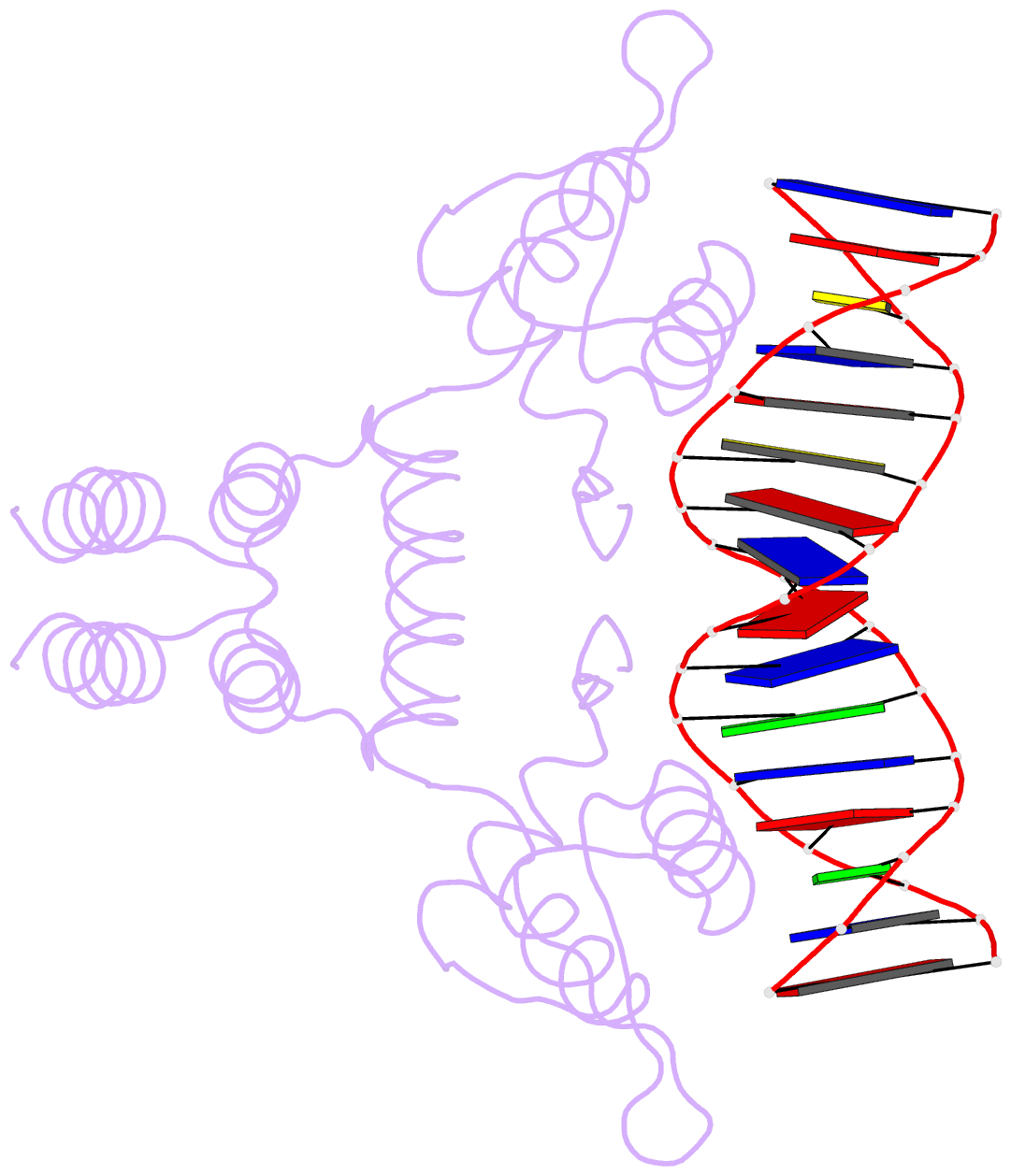
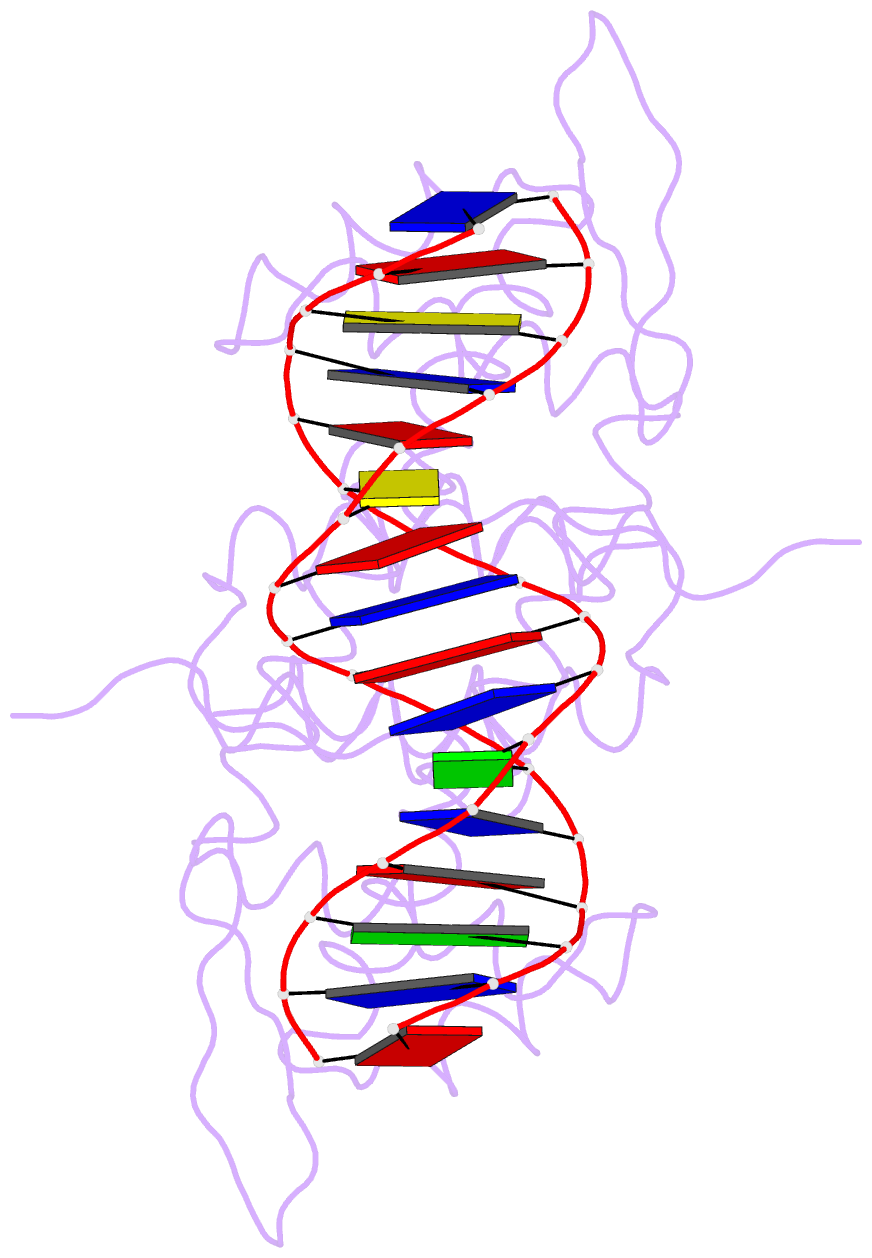
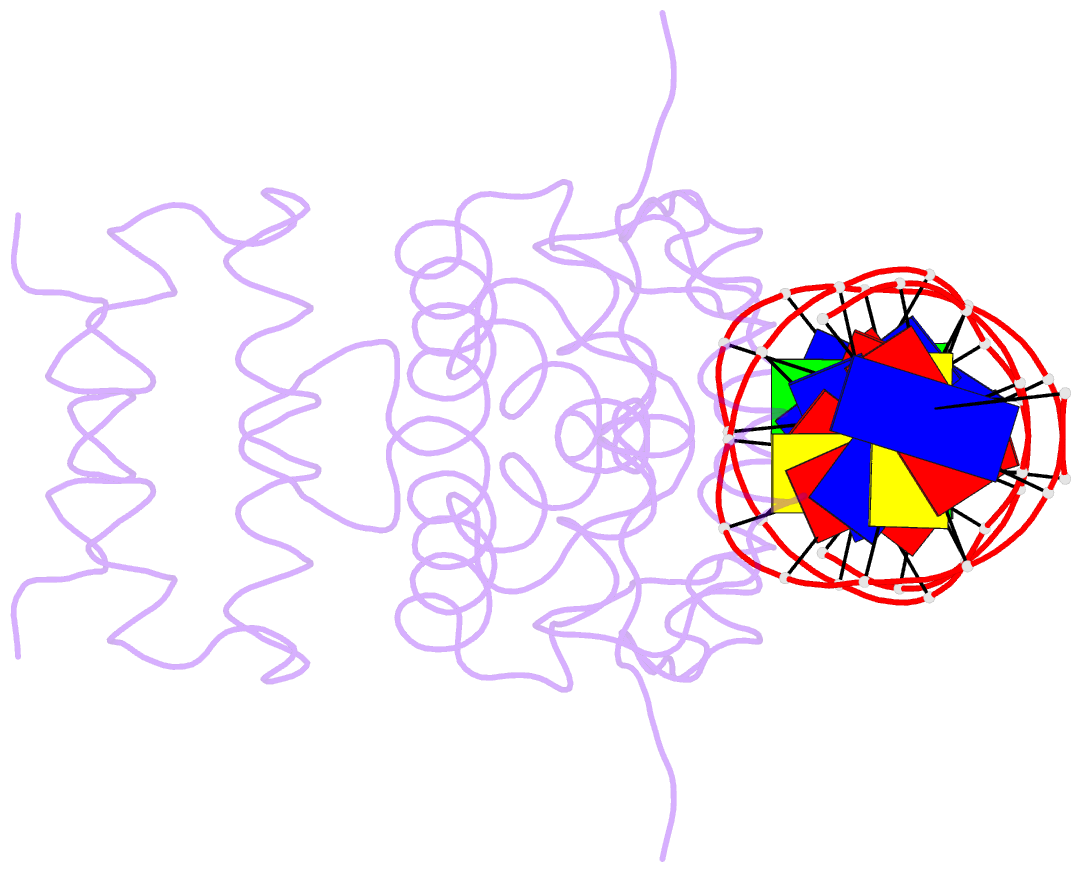
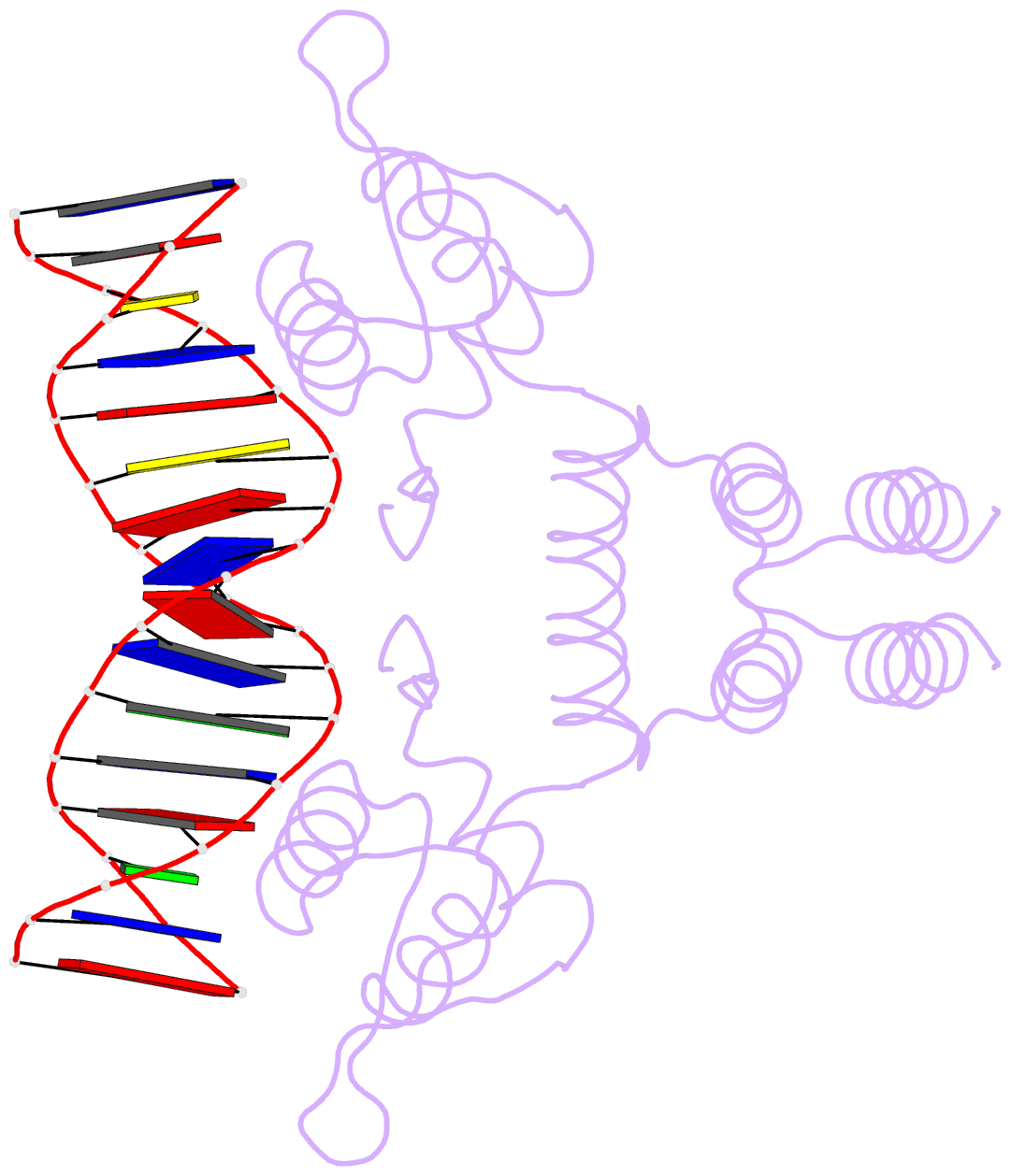
- Crystal structure of the blai repressor in complex with DNA
- Reference
- Safo MK, Zhao Q, Ko T-P, Musayev FN, Robinson H, Scarsdale N, Wang AH-J, Archer GL (2005): "Crystal structures of the BlaI repressor from Staphylococcus aureus and its complex with DNA: insights into transcriptional regulation of the bla and mec operons." J.Bacteriol., 187, 1833-1844. doi: 10.1128/JB.187.5.1833-1844.2005.
- Abstract
- The 14-kDa BlaI protein represses the transcription of blaZ, the gene encoding beta-lactamase. It is homologous to MecI, which regulates the expression of mecA, the gene encoding the penicillin binding protein PBP2a. These genes mediate resistance to beta-lactam antibiotics in staphylococci. Both repressors can bind either bla or mec DNA promoter-operator sequences. Regulated resistance genes are activated via receptor-mediated cleavage of the repressors. Cleavage is induced when beta-lactam antibiotics bind the extramembrane sensor of the sensor-transducer signaling molecules, BlaR1 or MecR1. The crystal structures of BlaI from Staphylococcus aureus, both in free form and in complex with 32 bp of DNA of the mec operator, have been determined to 2.0- and 2.7-A resolutions, respectively. The structure of MecI, also in free form and in complex with the bla operator, has been previously reported. Both repressors form homodimers, with each monomer composed of an N-terminal DNA binding domain of winged helix-turn-helix topology and a C-terminal dimerization domain. The structure of BlaI in complex with the mec operator shows a protein-DNA interface that is conserved between both mec and bla targets. The recognition helix alpha3 interacts specifically with the conserved TACA/TGTA DNA binding motif. BlaI and, probably, MecI dimers bind to opposite faces of the mec DNA double helix in an up-and-down arrangement, whereas MecI and, probably, BlaI dimers bind to the same DNA face of bla promoter-operator DNA. This is due to the different spacing of mec and bla DNA binding sites. Furthermore, the flexibility of the dimeric proteins may make the C-terminal proteolytic cleavage site more accessible when the repressors are bound to DNA than when they are in solution, suggesting that the induction cascade involves bound rather than free repressor.