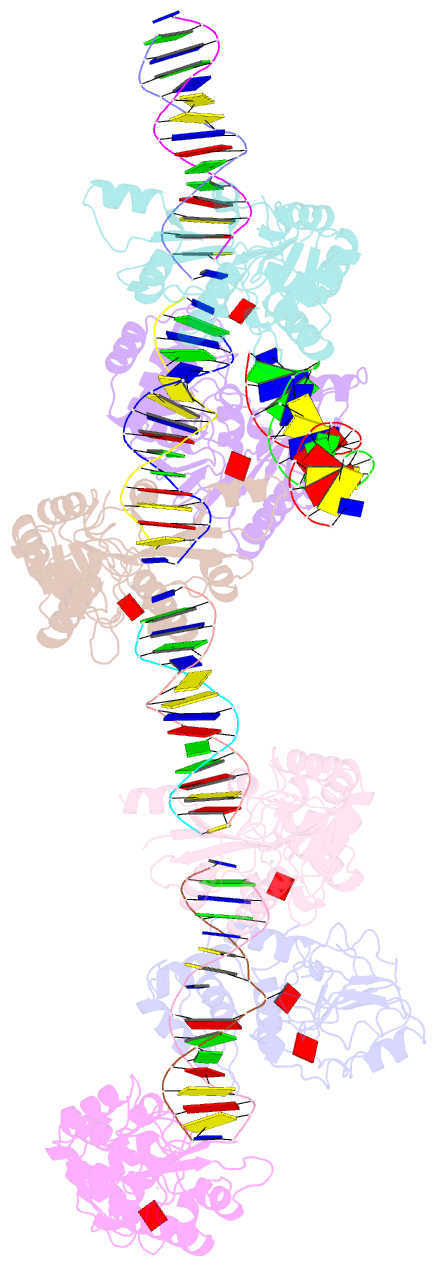
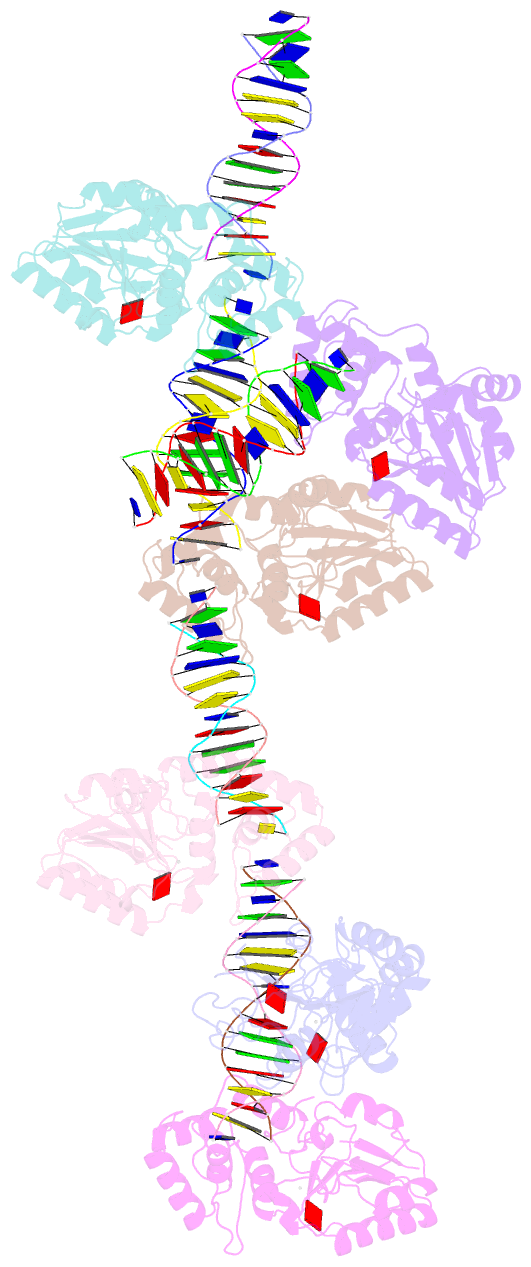
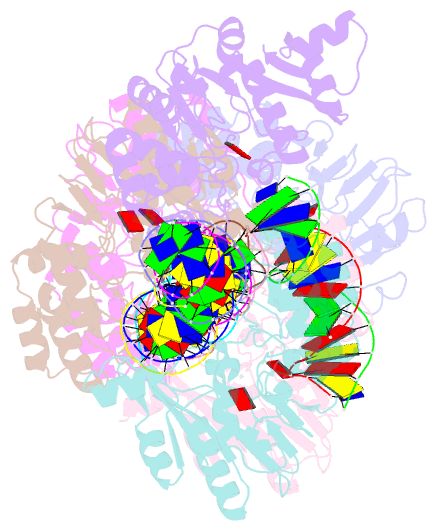
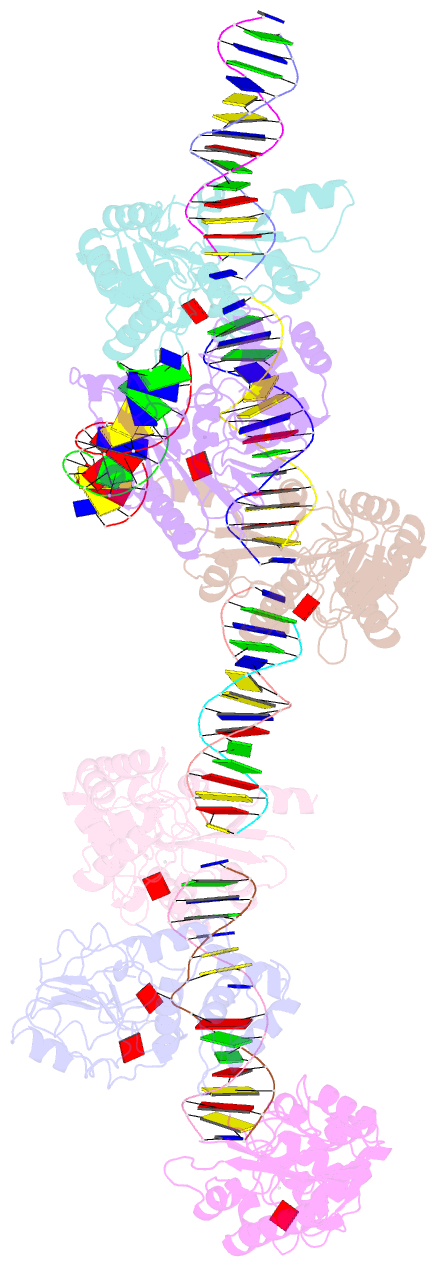
Summary information and primary citation
- PDB-id
-
1yfj;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.69 Å)
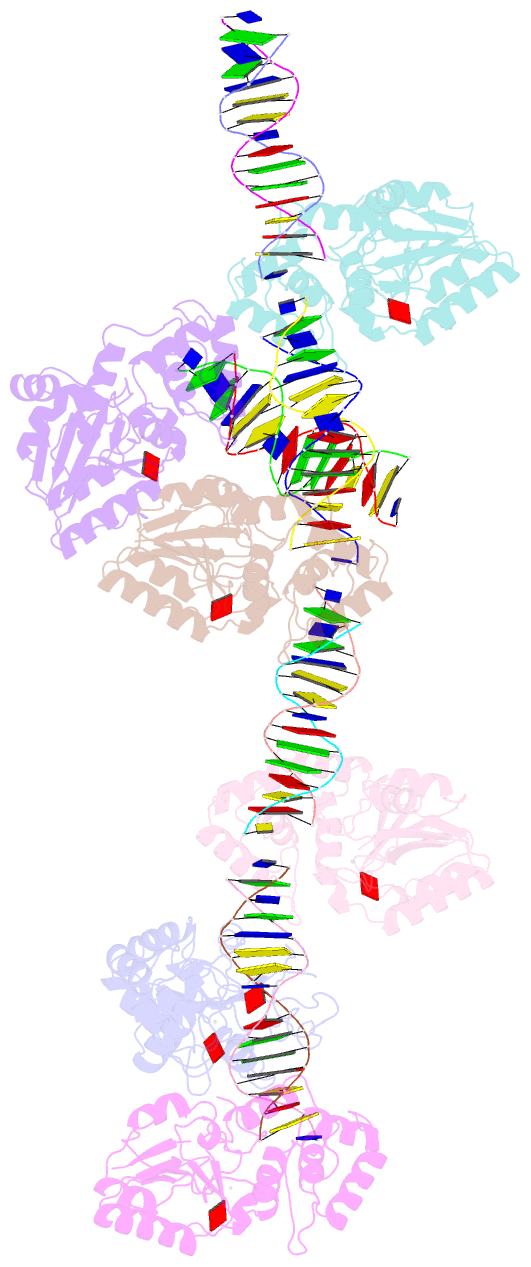
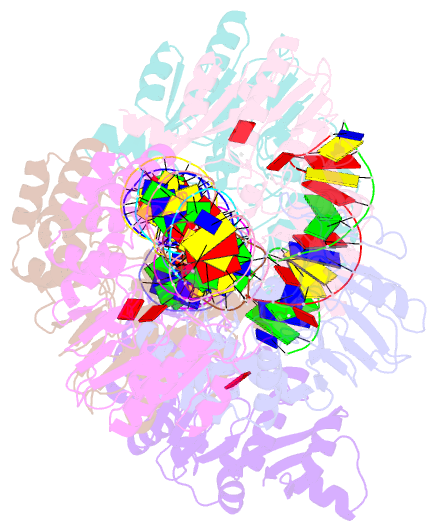
- Summary
- T4dam in complex with adohcy and 15-mer oligonucleotide
showing semi-specific and specific contact
- Reference
-
Horton JR, Liebert K, Hattman S, Jeltsch A, Cheng X
(2005): "Transition
from Nonspecific to Specific DNA Interactions along the
Substrate-Recognition Pathway of Dam
Methyltransferase." Cell(Cambridge,Mass.),
121, 349-361. doi: 10.1016/j.cell.2005.02.021.
- Abstract
- DNA methyltransferases methylate target bases within
specific nucleotide sequences. Three structures are
described for bacteriophage T4 DNA-adenine
methyltransferase (T4Dam) in ternary complexes with
partially and fully specific DNA and a methyl-donor analog.
We also report the effects of substitutions in the related
Escherichia coli DNA methyltransferase (EcoDam), altering
residues corresponding to those involved in specific
interaction with the canonical GATC target sequence in
T4Dam. We have identified two types of protein-DNA
interactions: discriminatory contacts, which stabilize the
transition state and accelerate methylation of the cognate
site, and antidiscriminatory contacts, which do not
significantly affect methylation of the cognate site but
disfavor activity at noncognate sites. These structures
illustrate the transition in enzyme-DNA interaction from
nonspecific to specific interaction, suggesting that there
is a temporal order for formation of specific
contacts.