Summary information and primary citation
- PDB-id
- 1yty; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-RNA
- Method
- X-ray (2.29 Å)
- Summary
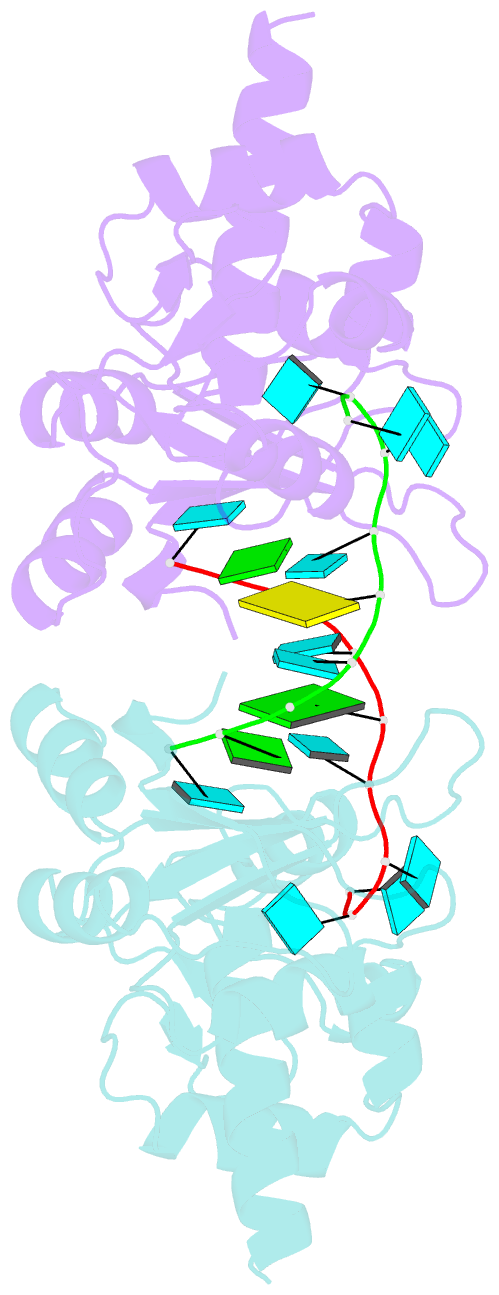
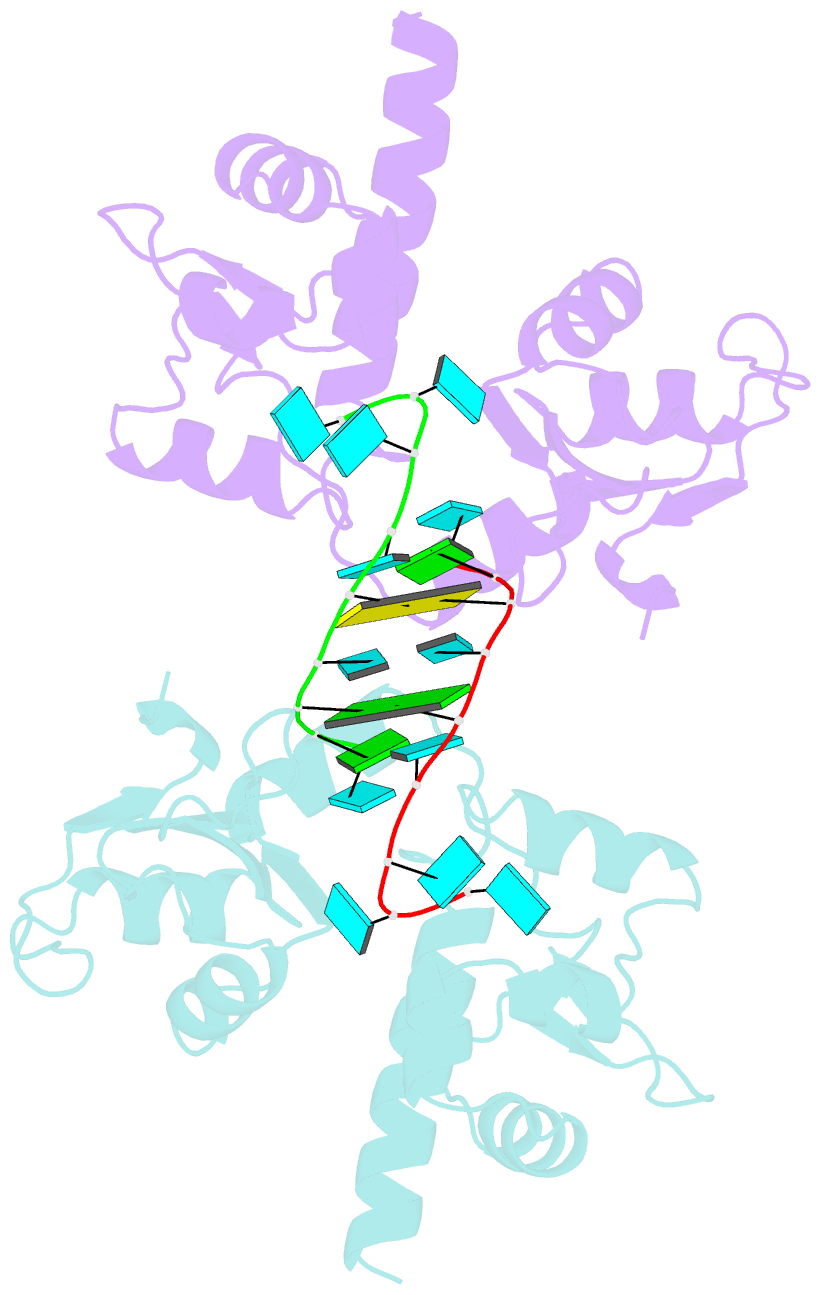
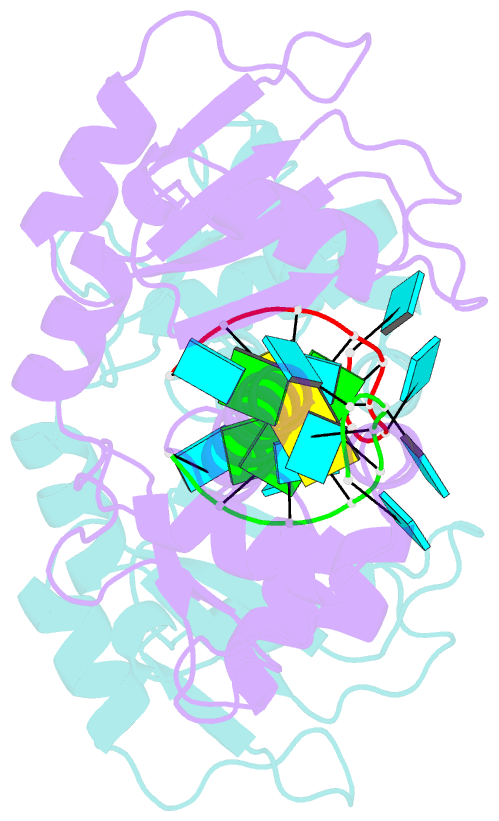
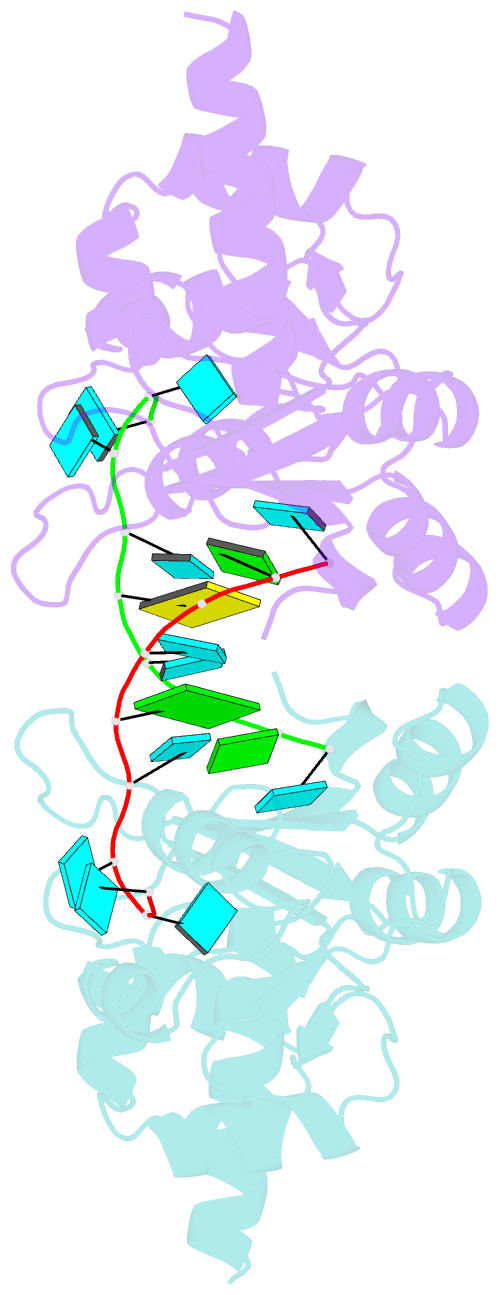
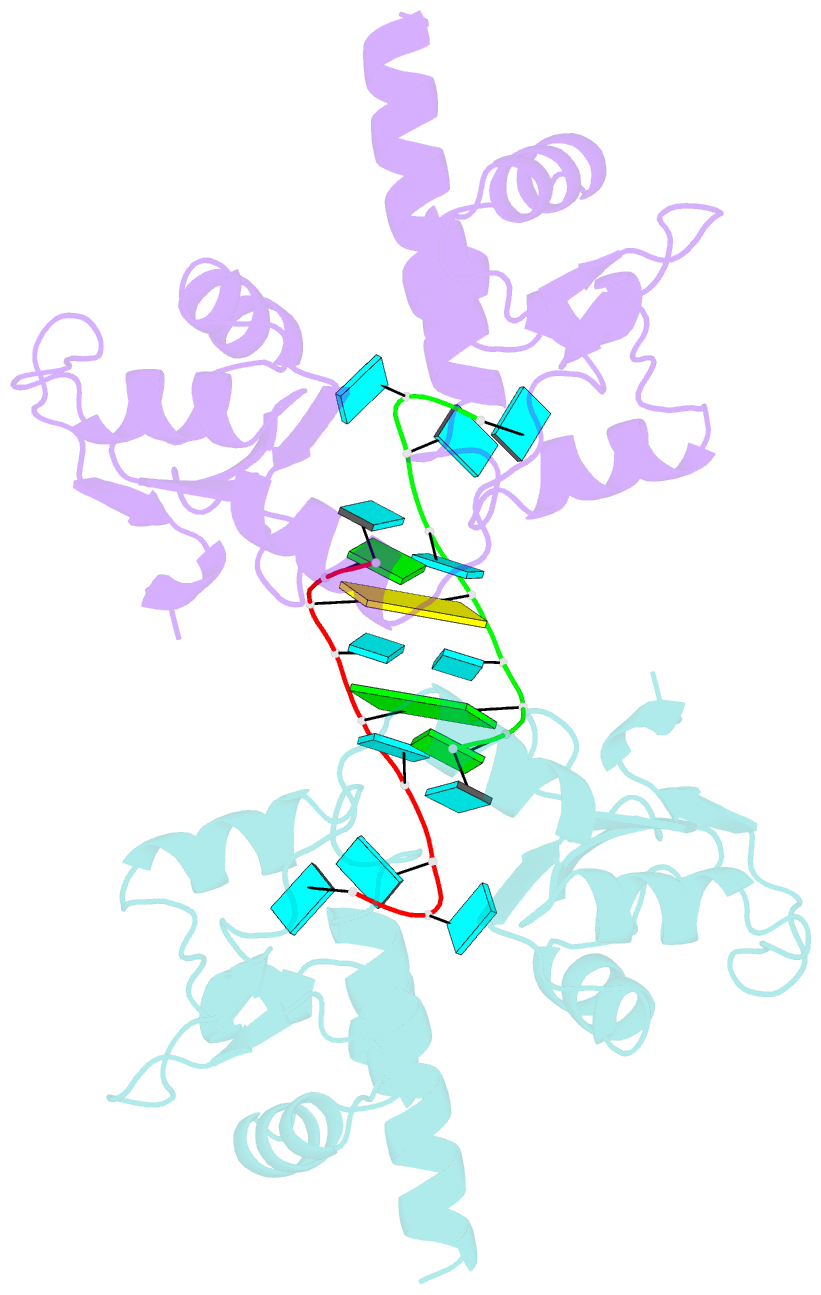
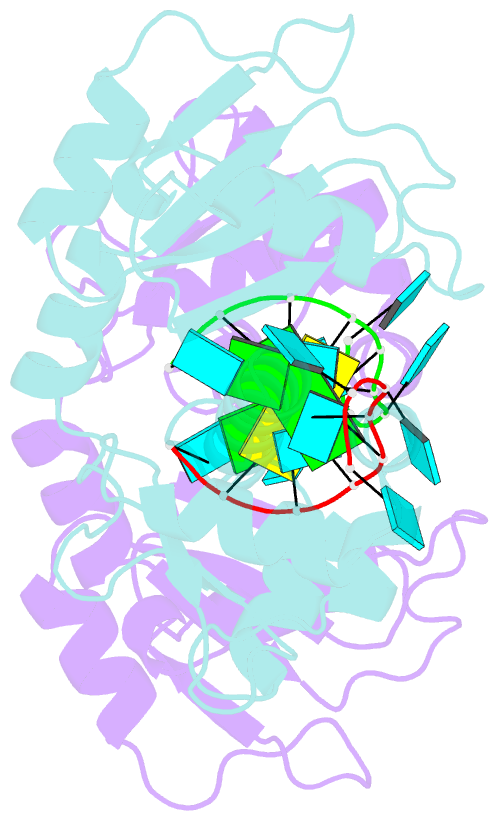
- Structural basis for recognition of uuuoh 3'-terminii of nascent RNA pol iii transcripts by la autoantigen
- Reference
- Teplova M, Yuan YR, Phan AT, Malinina L, Ilin S, Teplov A, Patel DJ (2006): "Structural Basis for Recognition and Sequestration of UUU(OH) 3' Temini of Nascent RNA Polymerase III Transcripts by La, a Rheumatic Disease Autoantigen." Mol.Cell, 21, 75-85. doi: 10.1016/j.molcel.2005.10.027.
- Abstract
- The nuclear phosphoprotein La was identified as an autoantigen in patients with systemic lupus erythematosus and Sjogren's syndrome. La binds to and protects the UUU(OH) 3' terminii of nascent RNA polymerase III transcripts from exonuclease digestion. We report the 1.85 angstroms crystal structure of the N-terminal domain of human La, consisting of La and RRM1 motifs, bound to r(U1-G2-C3-U4-G5-U6-U7-U8-U9OH). The U7-U8-U9OH 3' end, in a splayed-apart orientation, is sequestered within a basic and aromatic amino acid-lined cleft between the La and RRM1 motifs. The specificity-determining U8 residue bridges both motifs, in part through unprecedented targeting of the beta sheet edge, rather than the anticipated face, of the RRM1 motif. Our structural observations, supported by mutation studies of both La and RNA components, illustrate the principles behind RNA sequestration by a rheumatic disease autoantigen, whereby the UUU(OH) 3' ends of nascent RNA transcripts are protected during downstream processing and maturation events.