Summary information and primary citation
- PDB-id
- 1zx4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- cell cycle
- Method
- X-ray (2.98 Å)
- Summary
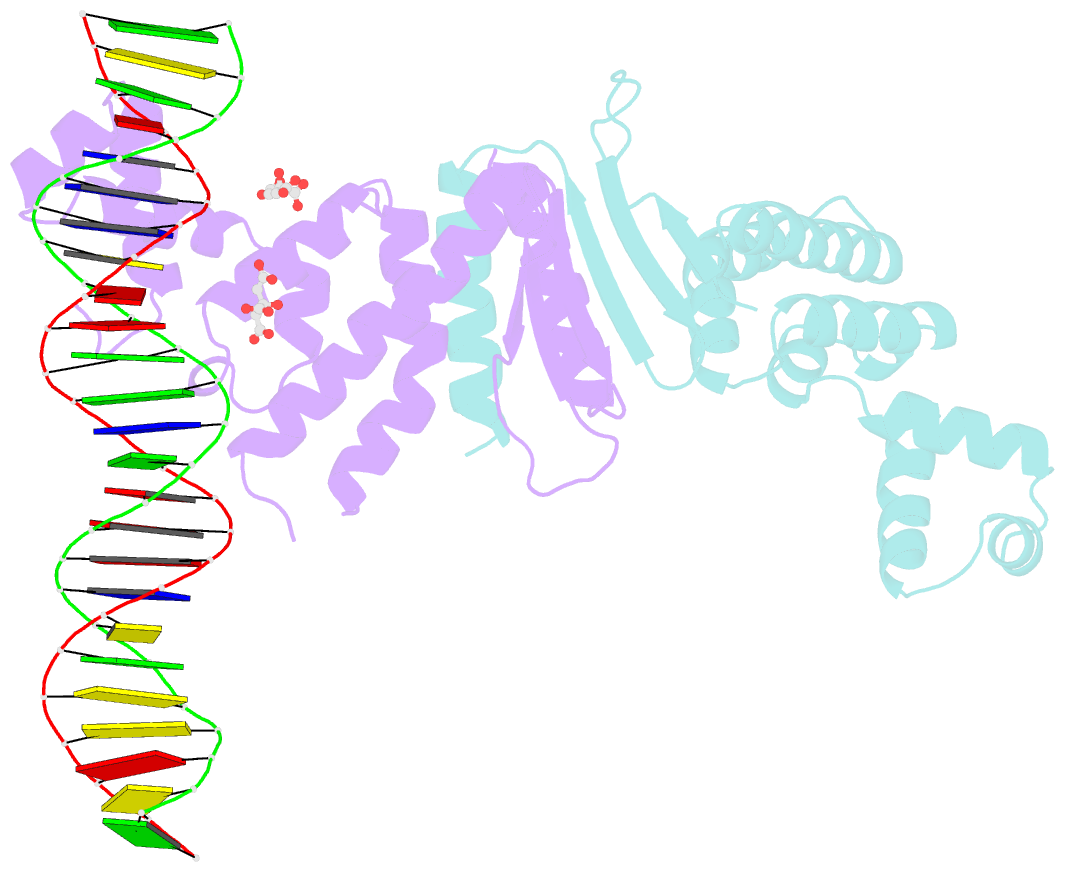
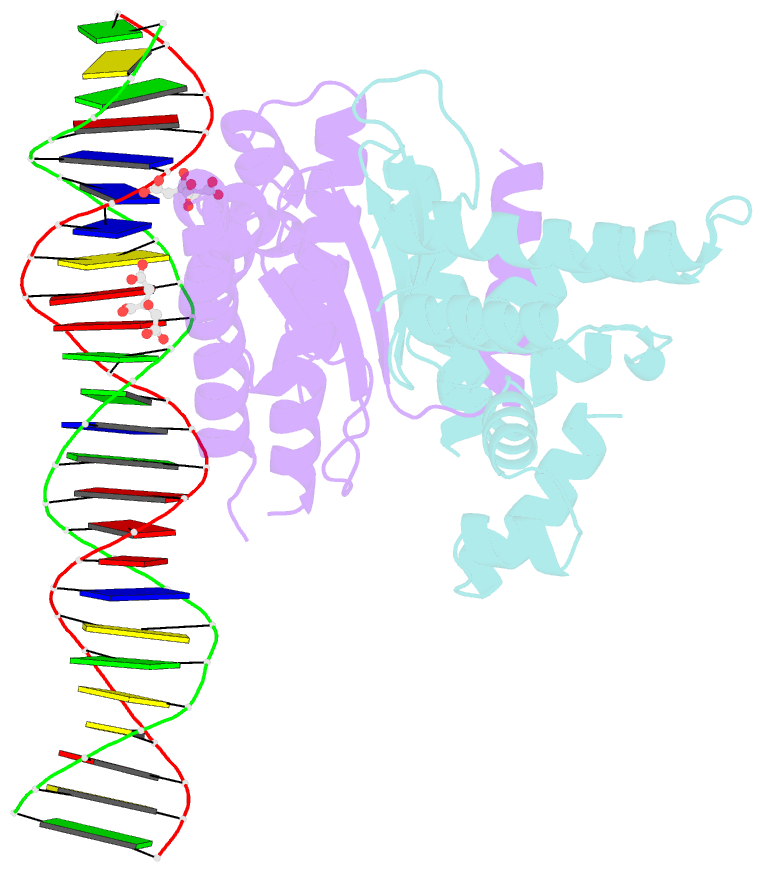
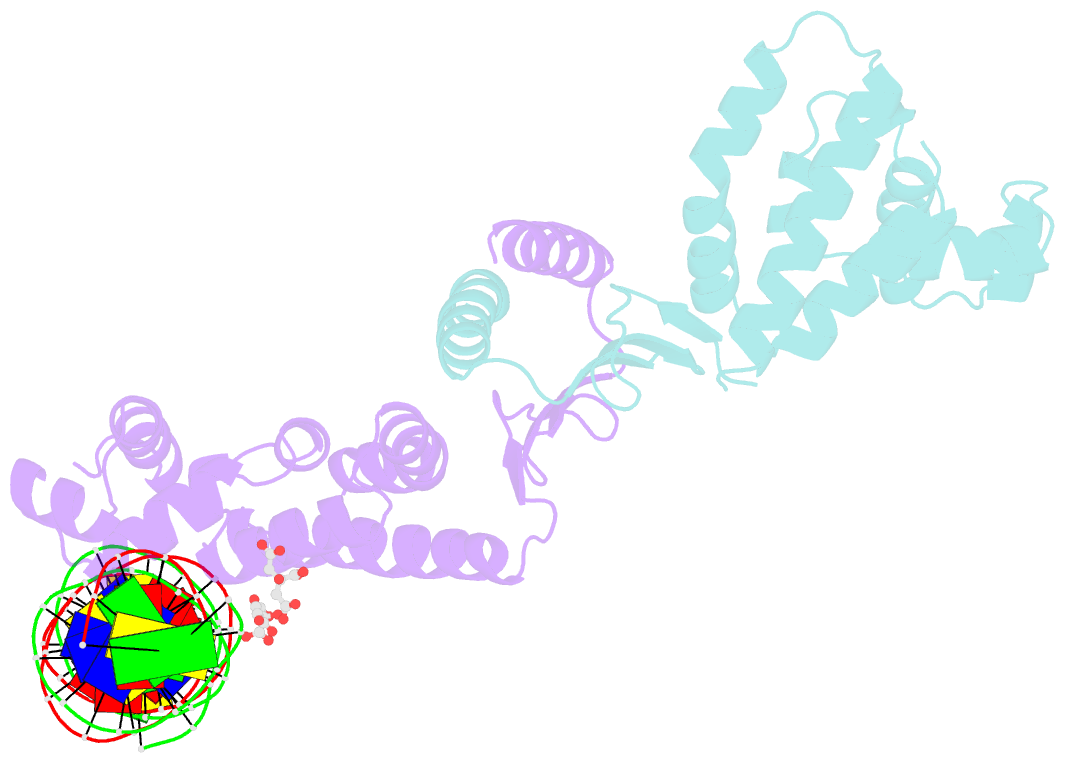
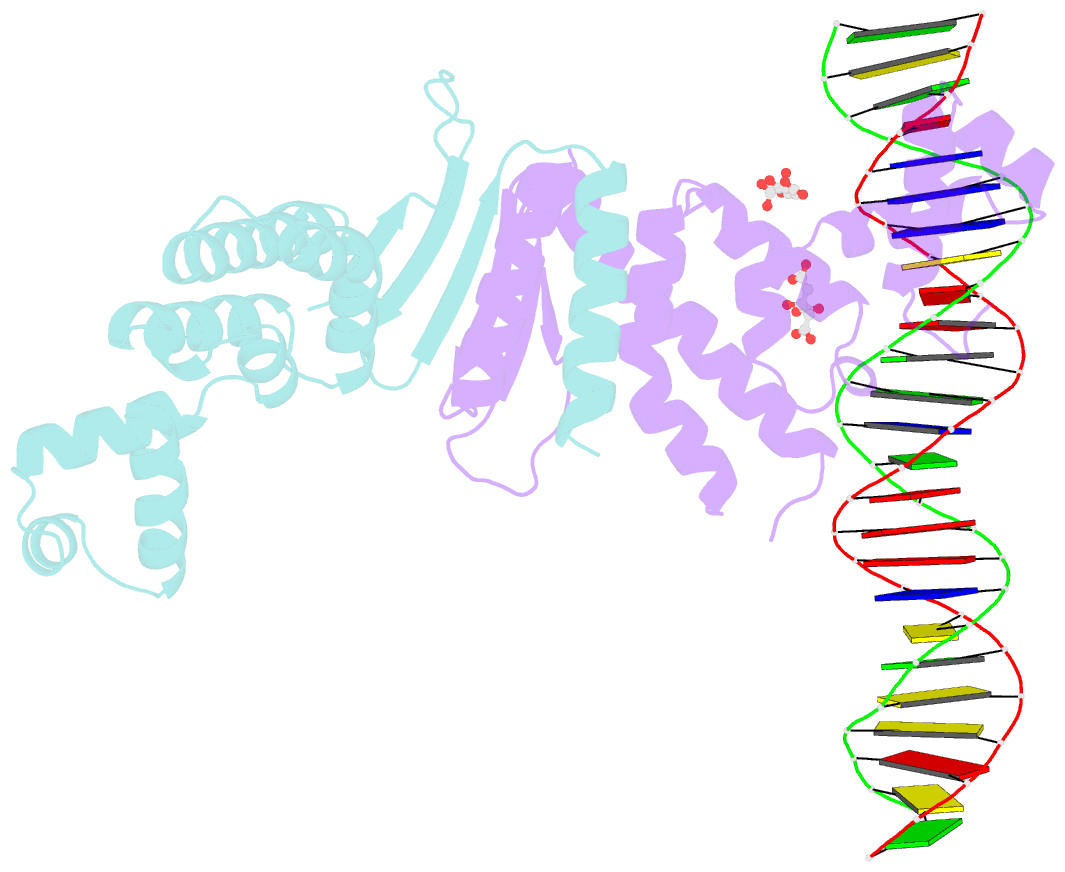
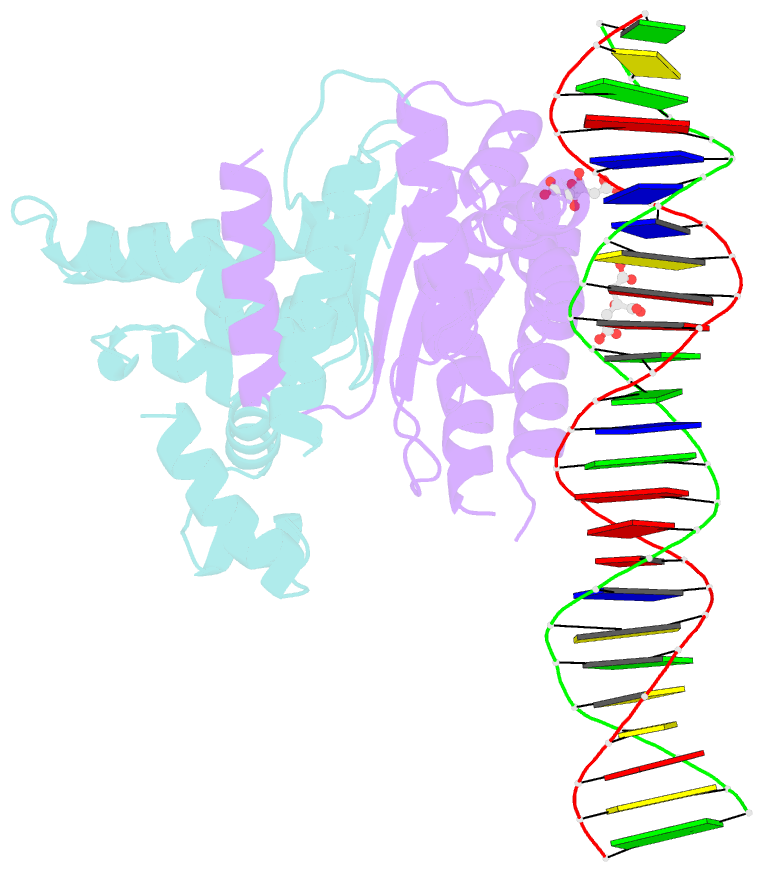
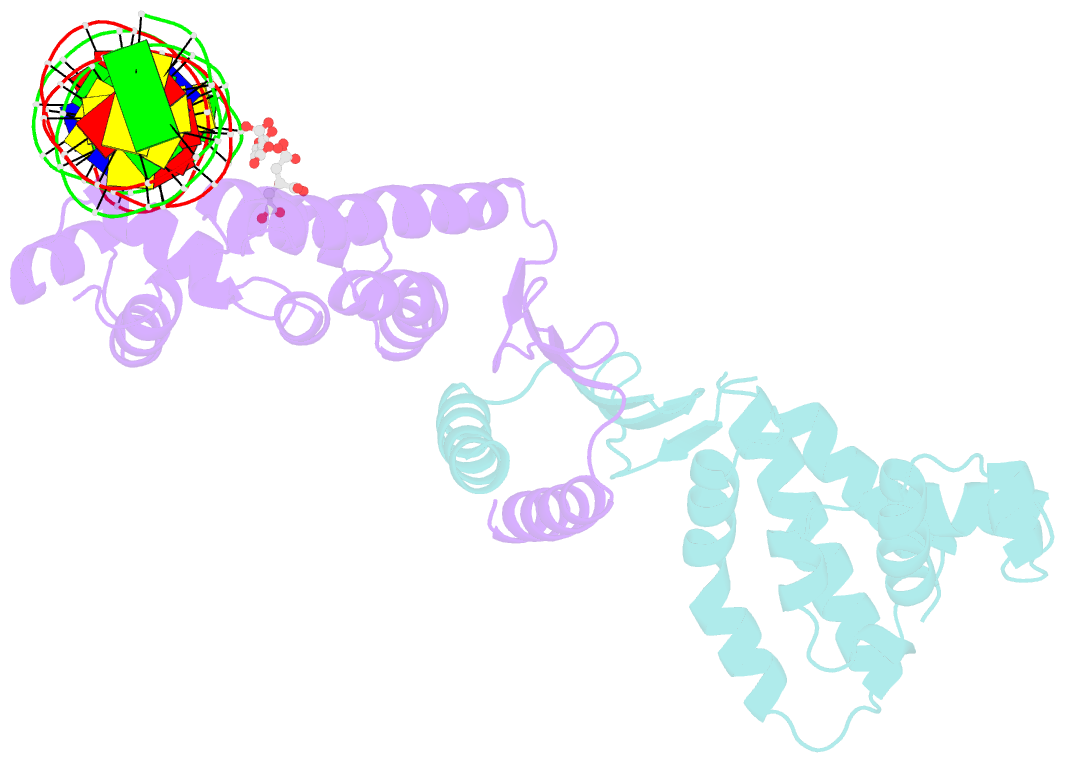
- Structure of parb bound to DNA
- Reference
- Schumacher MA, Funnell BE (2005): "Structures of ParB bound to DNA reveal mechanism of partition complex formation." Nature, 438, 516-519. doi: 10.1038/nature04149.
- Abstract
- The faithful inheritance of genetic information, which is essential for all organisms, requires accurate DNA partition (segregation) at cell division. In prokaryotes, partition is mediated by par systems, for which the P1 plasmid system of Escherichia coli is a prototype comprising a partition site and two proteins, ParA and ParB. To form the partition complex necessary for segregation, P1 ParB must recognize a complicated arrangement of A-box and B-box DNA motifs located on opposite ends of a sharply bent parS partition site of approximately 74 bp (refs 3-7). Here we describe structures of ParB bound to partition sites. ParB forms an asymmetric dimer with extended amino-terminal HTH (helix-turn-helix) domains that contact A-boxes. The two HTH domains emanate from a dimerized DNA-binding module composed of a six-stranded beta-sheet coiled-coil that binds B-boxes. Strikingly, these individual DNA-binding modules rotate freely about a flexible linker, enabling them to contact several arrangements of A- and B-boxes. Most notably, each DNA-binding element binds to and thus bridges adjacent DNA duplexes. These unique structural features of ParB explain how this protein can bind complex arrays of A- and B-box elements on adjacent DNA arms of the looped partition site.