Summary information and primary citation
- PDB-id
- 2a1r; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.6 Å)
- Summary
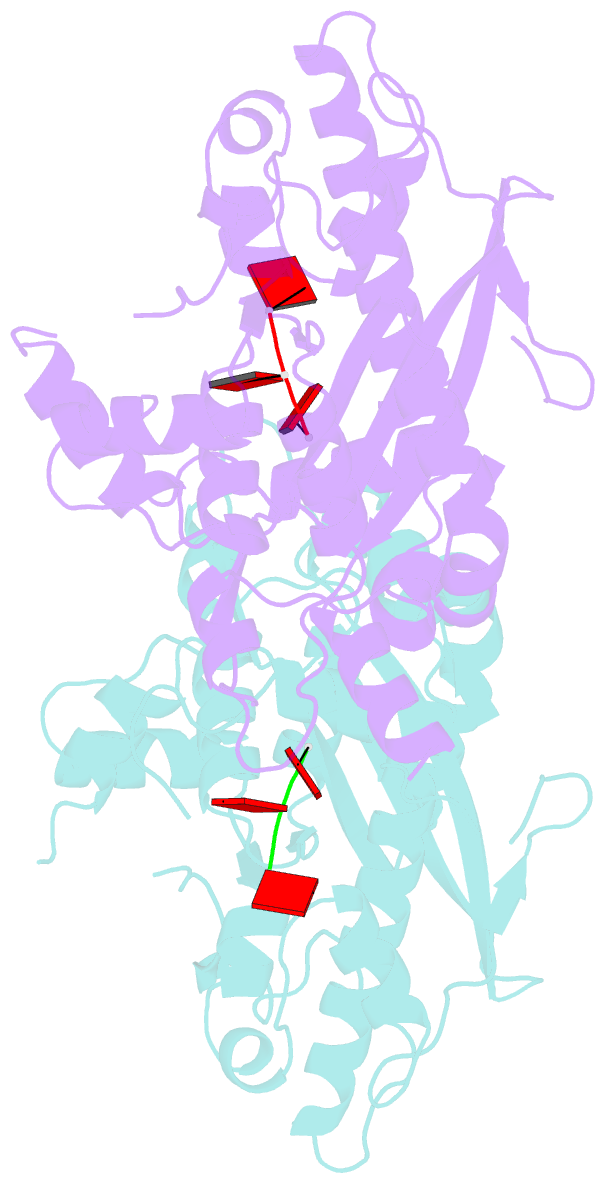
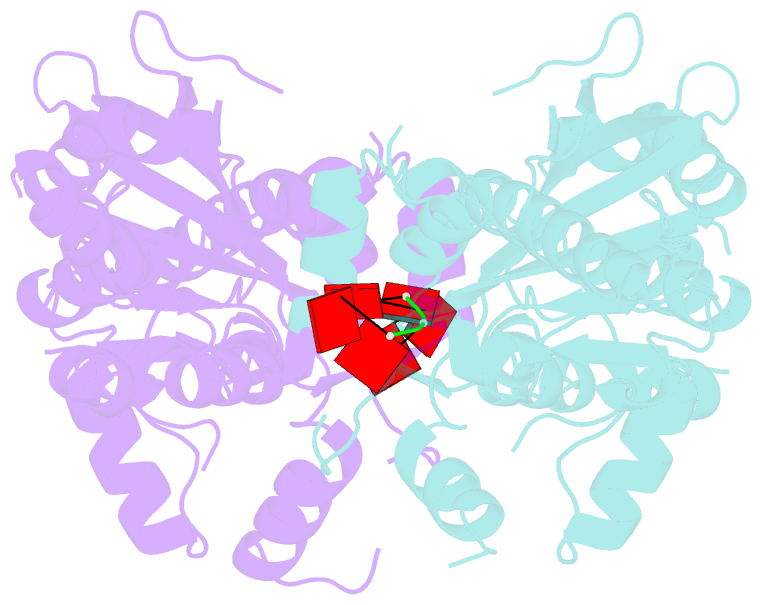
- Crystal structure of parn nuclease domain
- Reference
- Wu M, Reuter M, Lilie H, Liu Y, Wahle E, Song H (2005): "Structural insight into poly(A) binding and catalytic mechanism of human PARN." Embo J., 24, 4082-4093. doi: 10.1038/sj.emboj.7600869.
- Abstract
- Poly(A)-specific ribonuclease (PARN) is a processive, poly(A)-specific 3' exoribonuclease. The crystal structure of C-terminal truncated human PARN determined in two states (free and RNA-bound forms) reveals that PARNn is folded into two domains, an R3H domain and a nuclease domain similar to those of Pop2p and epsilon186. The high similarity of the active site structures of PARNn and epsilon186 suggests that they may have a similar catalytic mechanism. PARNn forms a tight homodimer, with the R3H domain of one subunit partially enclosing the active site of the other subunit and poly(A) bound in a deep cavity of its nuclease domain in a sequence-nonspecific manner. The R3H domain and, possibly, the cap-binding domain are involved in poly(A) binding but these domains alone do not appear to contribute to poly(A) specificity. Mutations disrupting dimerization abolish both the enzymatic and RNA-binding activities, suggesting that the PARN dimer is a structural and functional unit. The cap-binding domain may act in concert with the R3H domain to amplify the processivity of PARN.