Summary information and primary citation
- PDB-id
- 2bte; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase
- Method
- X-ray (2.9 Å)
- Summary
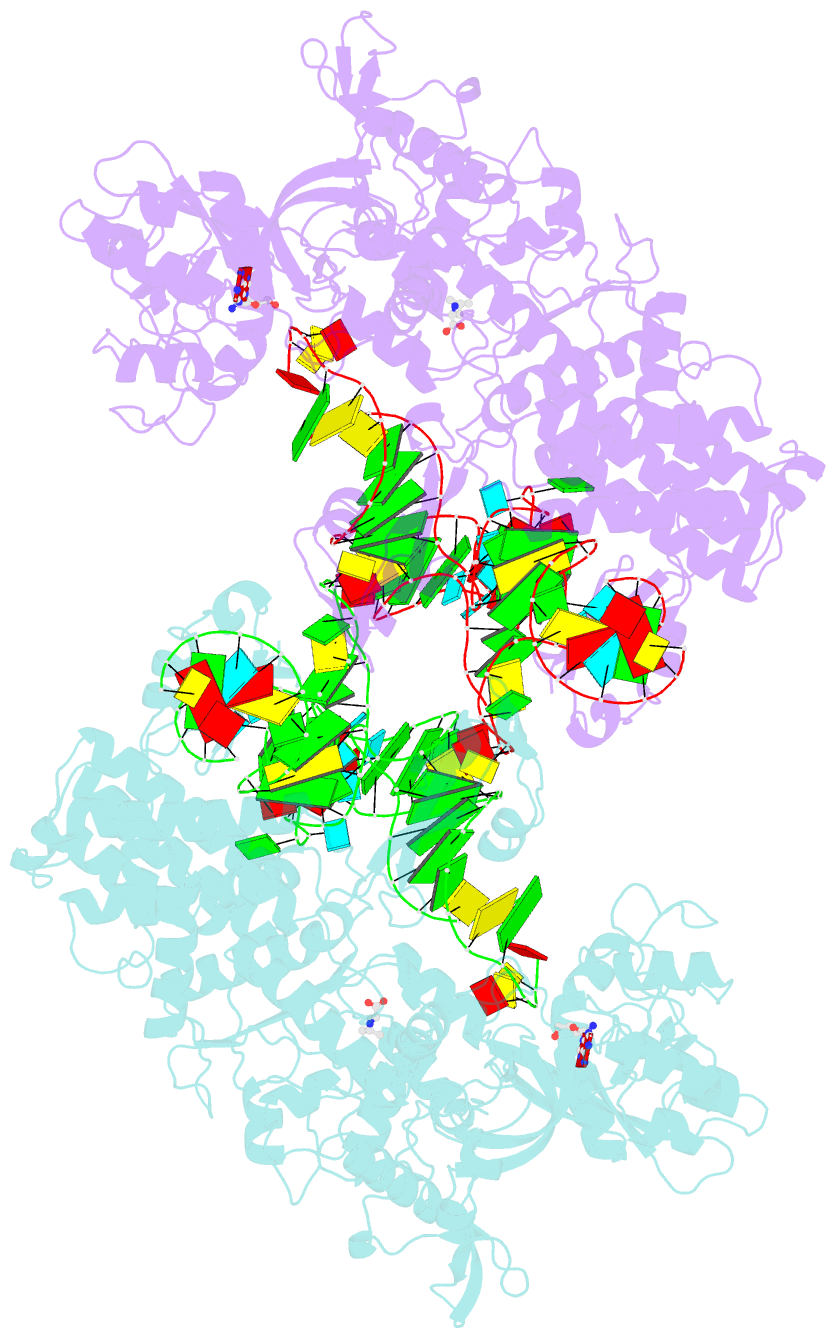
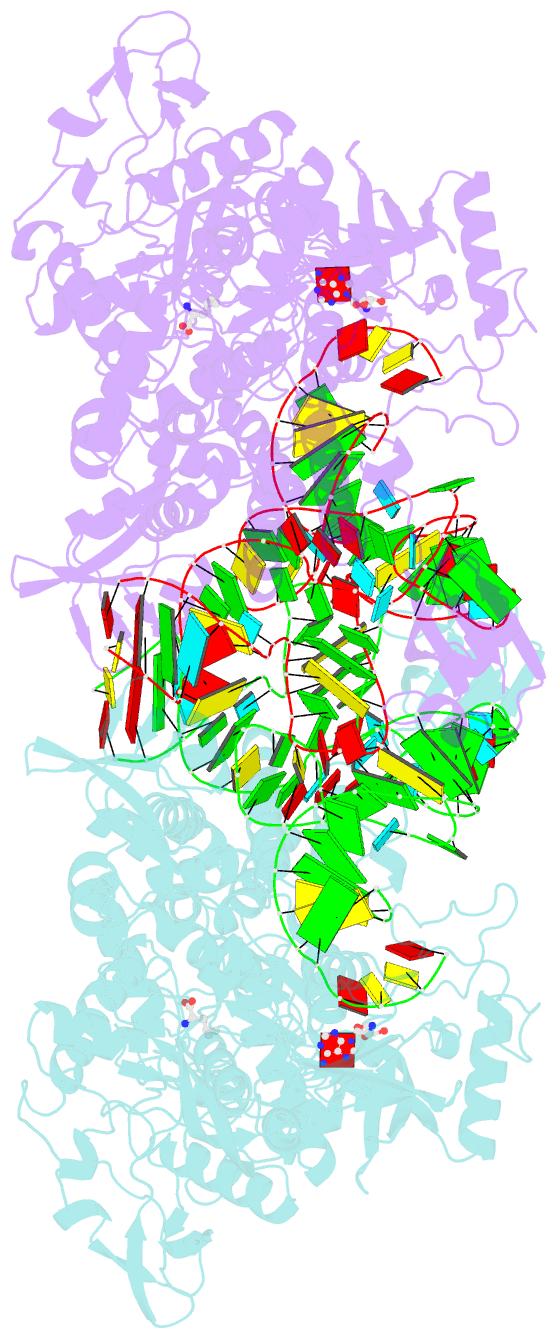
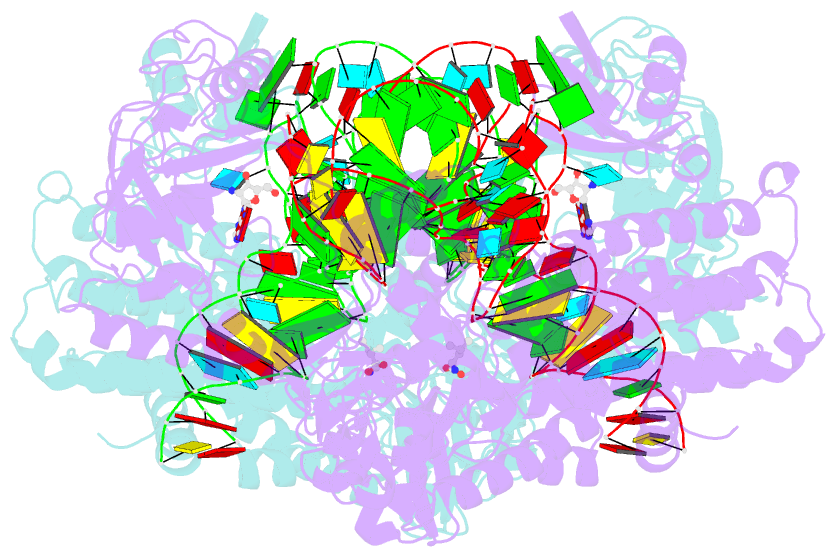
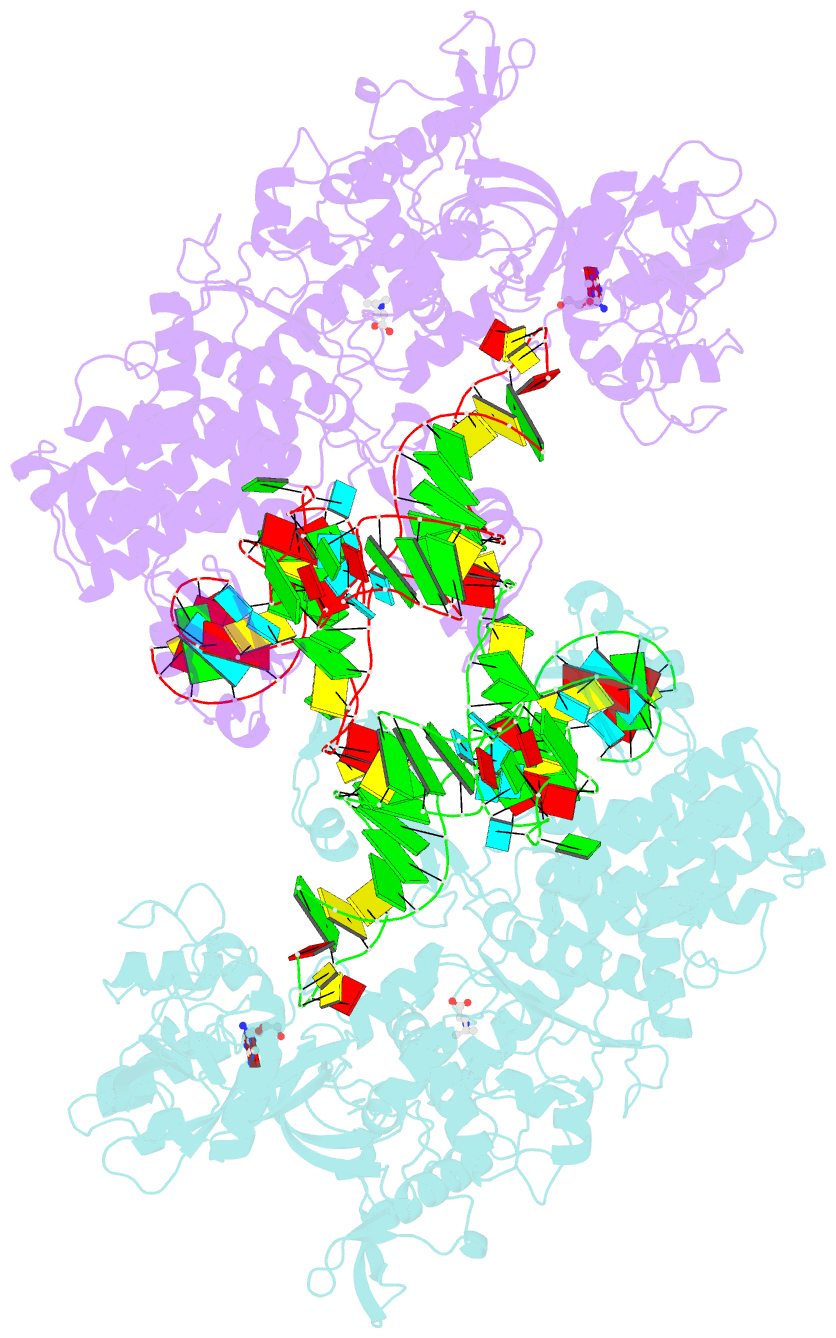
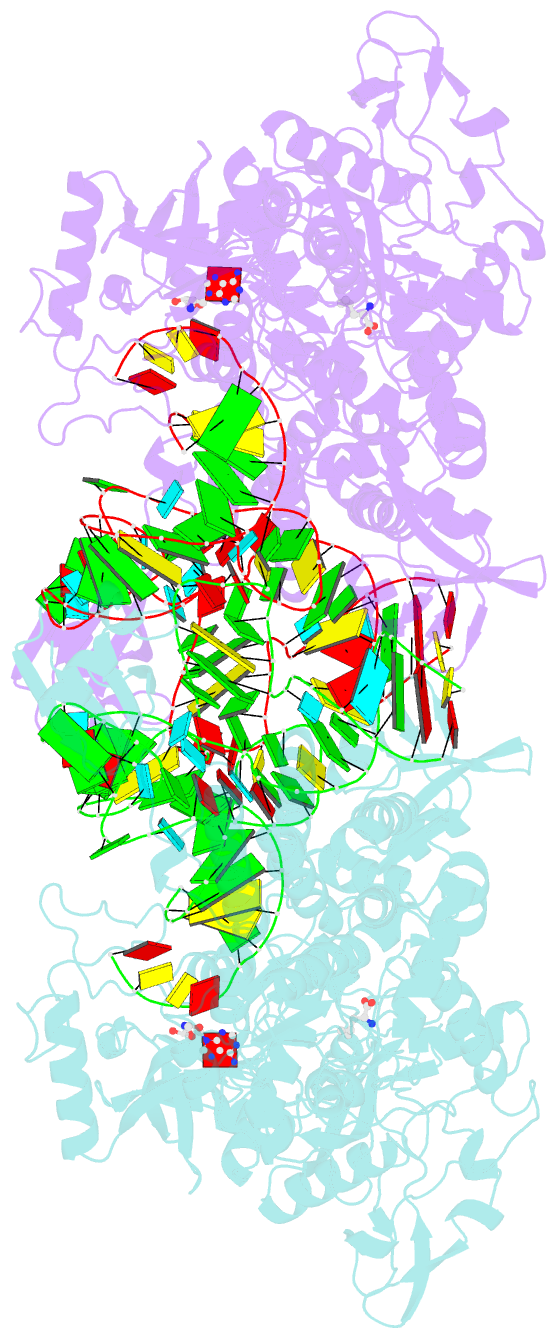
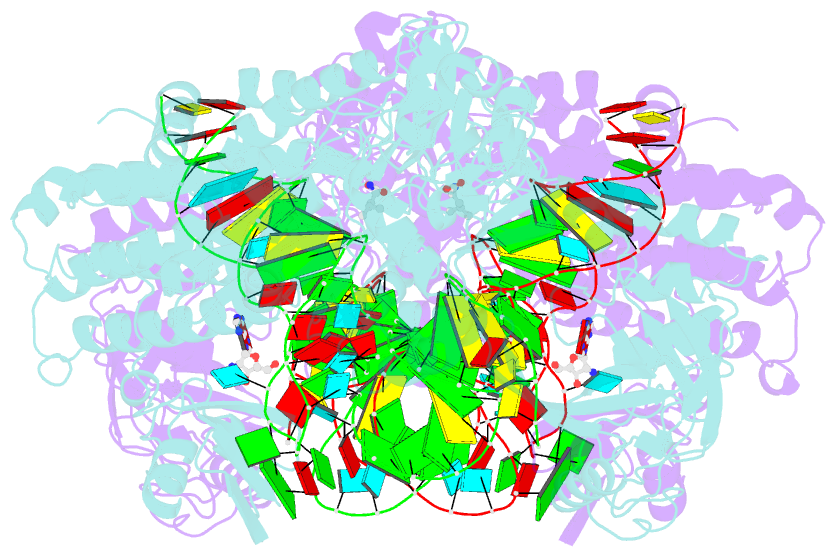
- Thermus thermophilus leucyl-trna synthetase complexed with a trnaleu transcript in the post-editing conformation and a post- transfer editing substrate analogue
- Reference
- Tukalo M, Yaremchuk A, Fukunaga R, Yokoyama S, Cusack S (2005): "The Crystal Structure of Leucyl-tRNA Synthetase Complexed with tRNA(Leu) in the Post-Transfer- Editing Conformation." Nat.Struct.Mol.Biol., 12, 923. doi: 10.1038/NSMB986.
- Abstract
- Leucyl-tRNA synthetase (LeuRS) has a specific post-transfer editing activity directed against mischarged isoleucine and similar noncognate amino acids. We describe the post-transfer-editing and product complexes of Thermus thermophilus LeuRS (LeuRSTT) with tRNA(Leu) at 2.9- to 3.3-A resolution. In the post-transfer-editing configuration, A76 binds in the editing active site exactly as previously found for the adenosine moiety of a small-molecule editing-substrate analog. The 60 C-terminal residues of LeuRSTT, unseen in previous structures, fold into a compact domain flexibly linked to the rest of the molecule and interacting with the G19-C56 tertiary base pair of tRNA(Leu). LeuRS recognition of tRNA(Leu) depends essentially on tRNA shape rather than base-specific interactions. The structures show that considerable domain rotations, notably of the editing domain, accompany the tRNA-3' end dynamics associated successively with aminoacylation, post-transfer editing and product release.