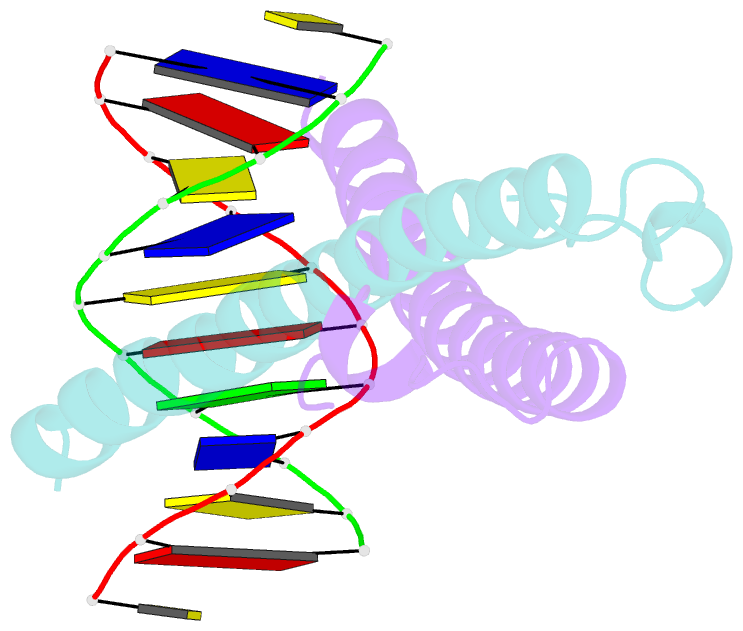
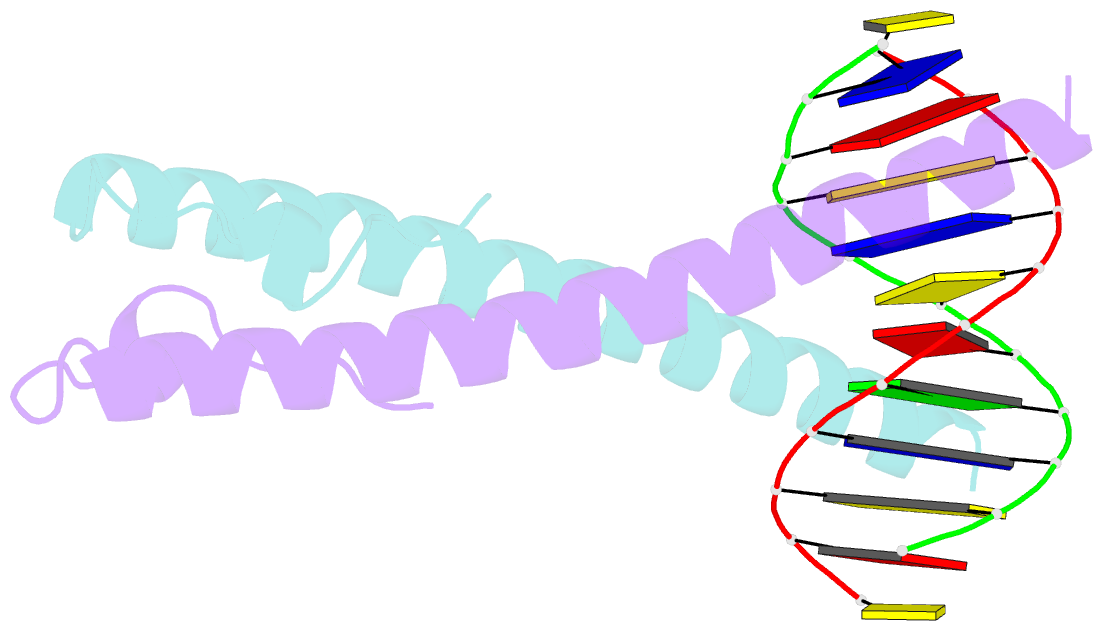
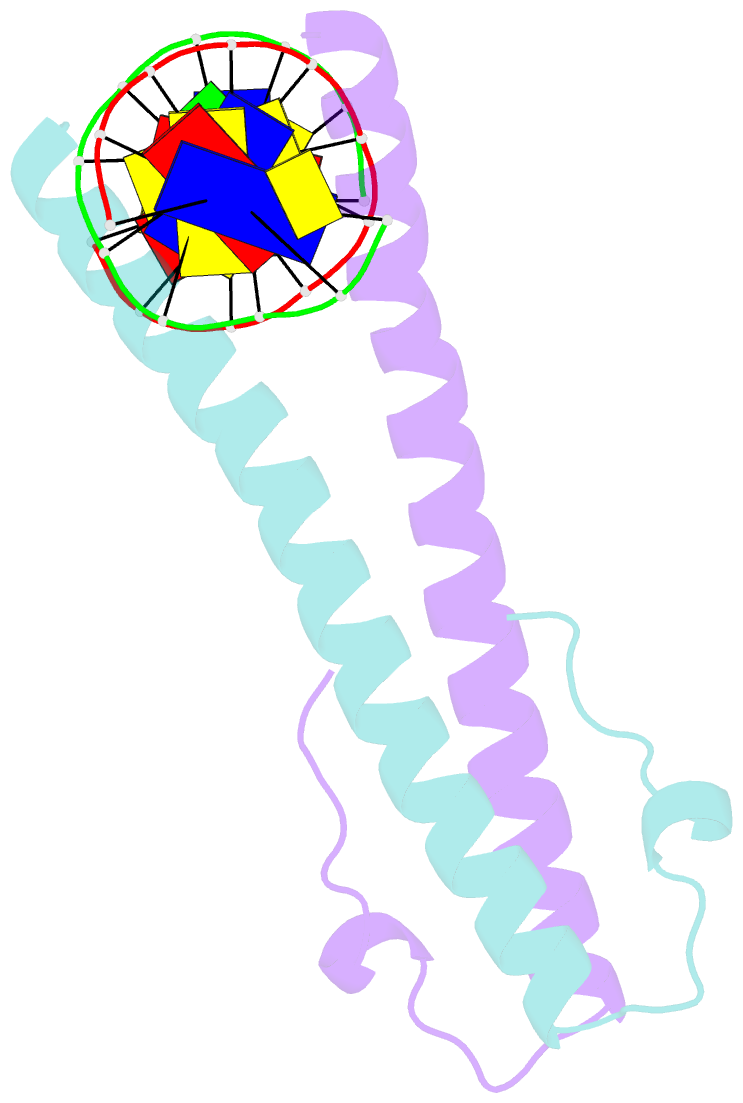
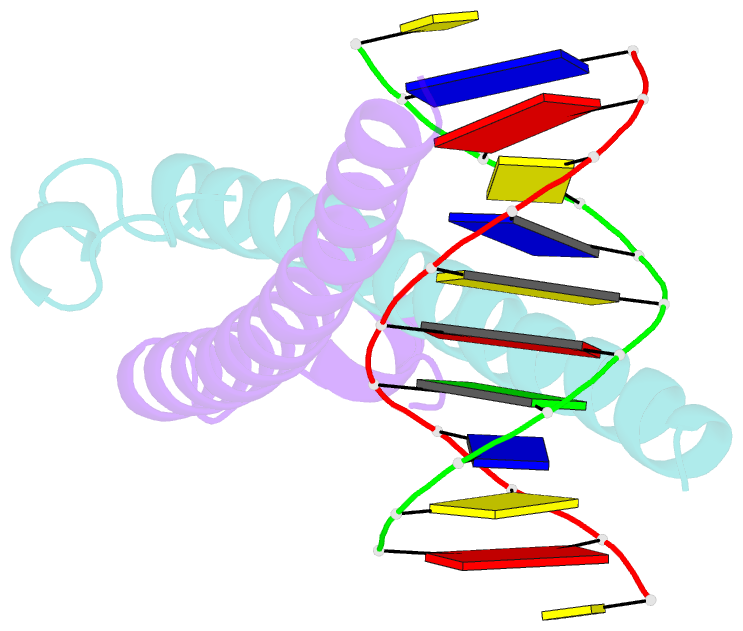
Summary information and primary citation
- PDB-id
- 2c9n; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- X-ray (3.3 Å)
- Summary
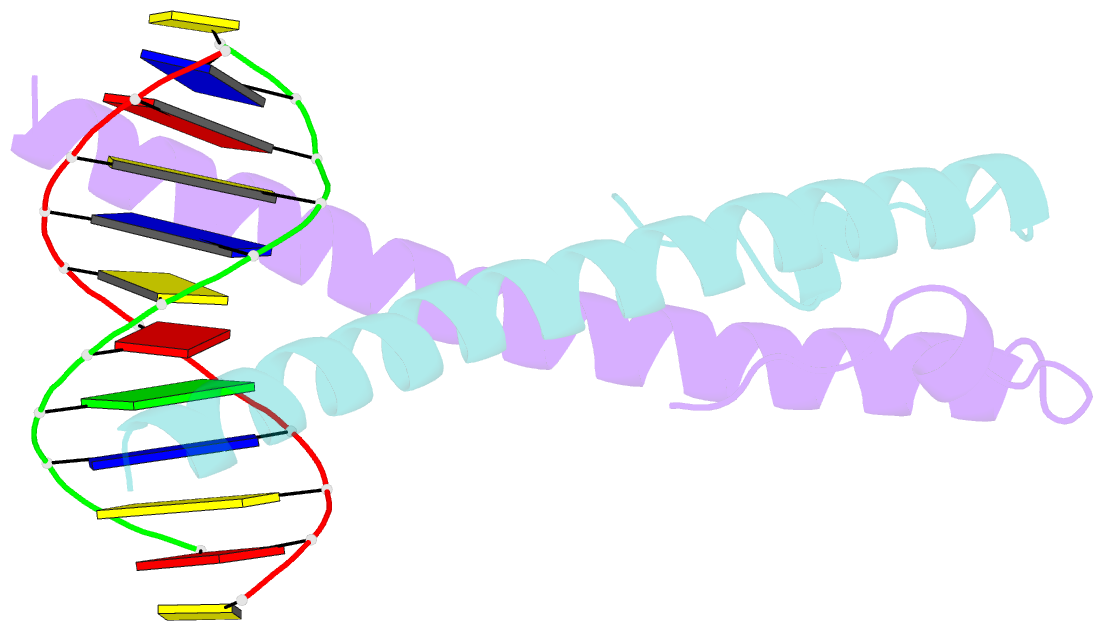
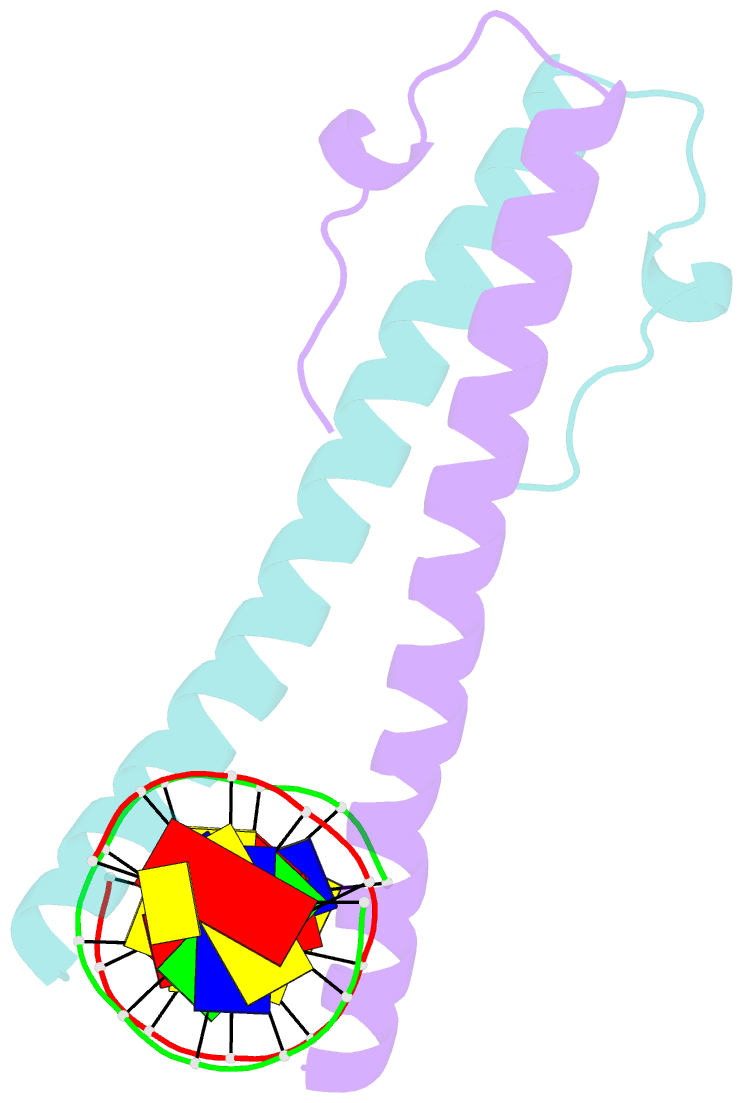
- Structure of the epstein-barr virus zebra protein at approximately 3. 5 angstrom resolution
- Reference
- Petosa C, Morand P, Baudin F, Moulin M, Artero JB, Muller CW (2006): "Structural Basis of Lytic Cycle Activation by the Epstein-Barr Virus Zebra Protein." Mol.Cell, 21, 565. doi: 10.1016/J.MOLCEL.2006.01.006.
- Abstract
- Epstein-Barr virus (EBV) causes infectious mononucleosis and is linked to several human malignancies. EBV has a biphasic infection cycle consisting of a latent and a lytic, replicative phase. The switch from latent to lytic infection is triggered by the EBV immediate-early transcription factor ZEBRA (BZLF1, Zta, Z, EB1). We present the crystal structure of ZEBRA's DNA binding domain bound to an EBV lytic gene promoter element. ZEBRA exhibits a variant of the basic-region leucine zipper (bZIP) fold in which a C-terminal moiety stabilizes the coiled coil involved in dimer formation. The structure provides insights into ZEBRA's broad target site specificity, preferential activation of specific EBV promoters in their methylated state, ability to dimerize despite lacking a leucine zipper motif, and failure to heterodimerize with cellular bZIP proteins. The structure will allow for the design of new therapeutic agents that block activation of the EBV lytic cycle.