Summary information and primary citation
- PDB-id
- 2ccz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA-replication
- Method
- X-ray (2.7 Å)
- Summary
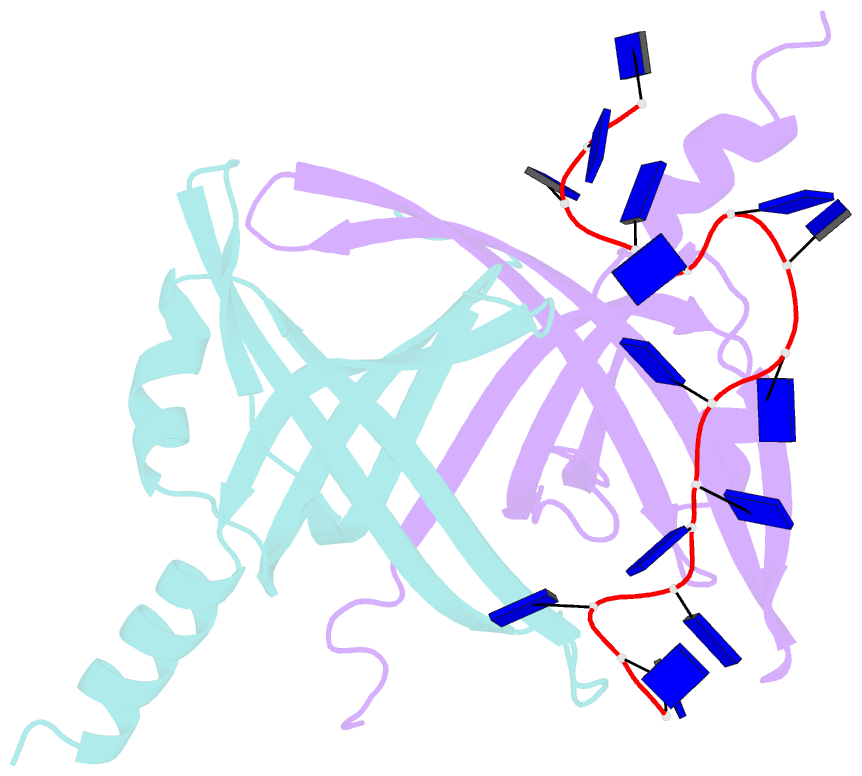
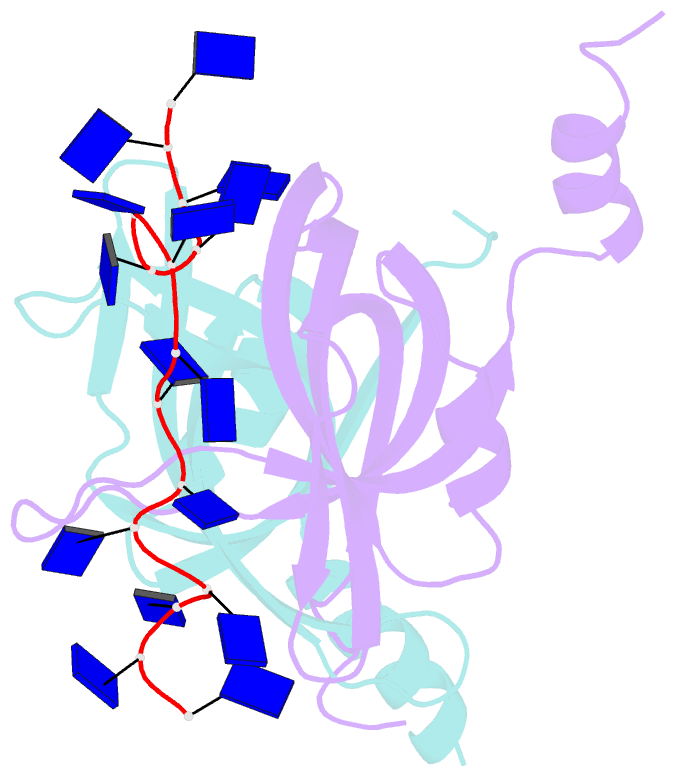
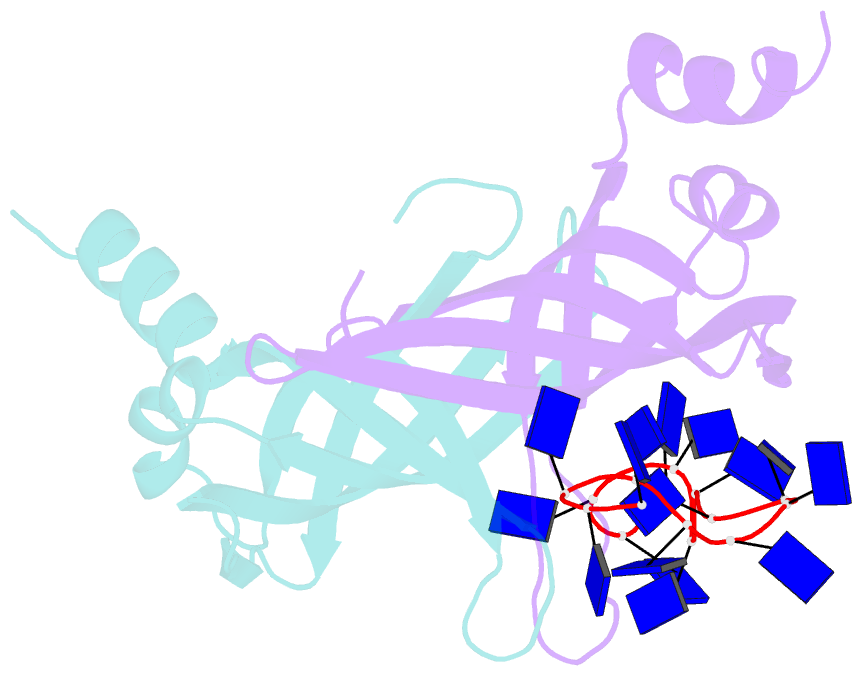
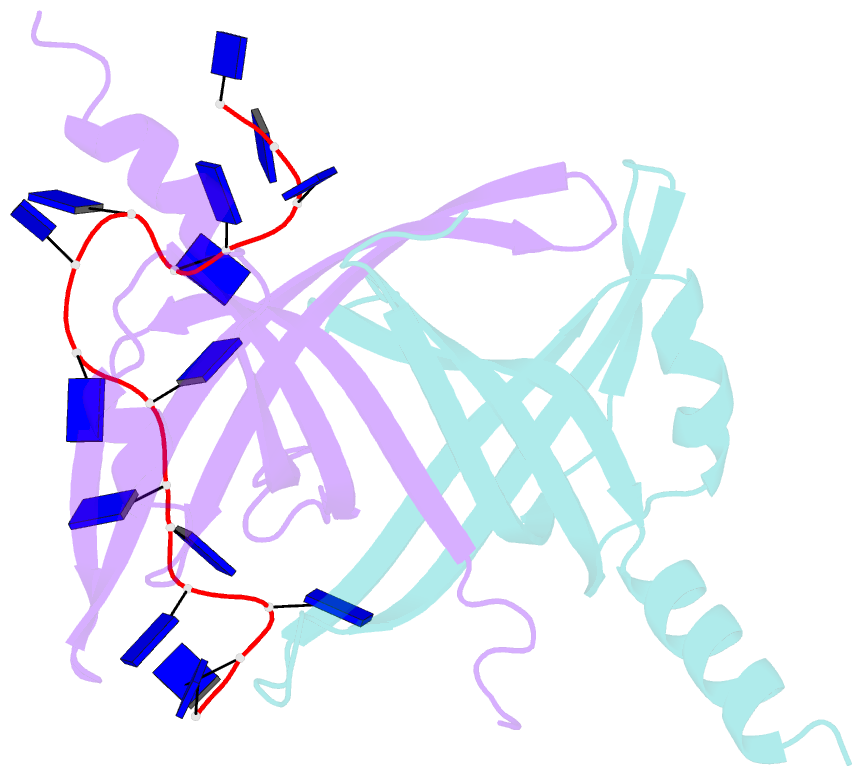
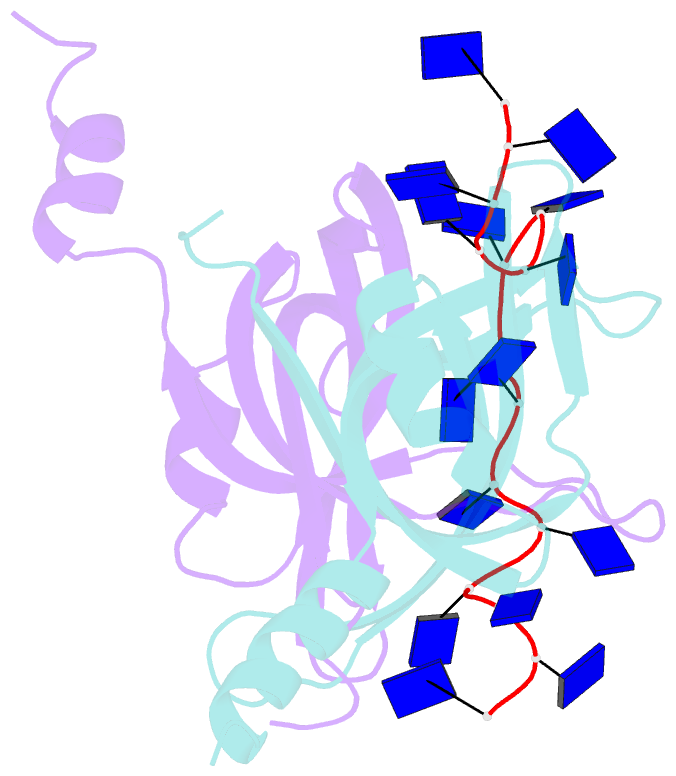
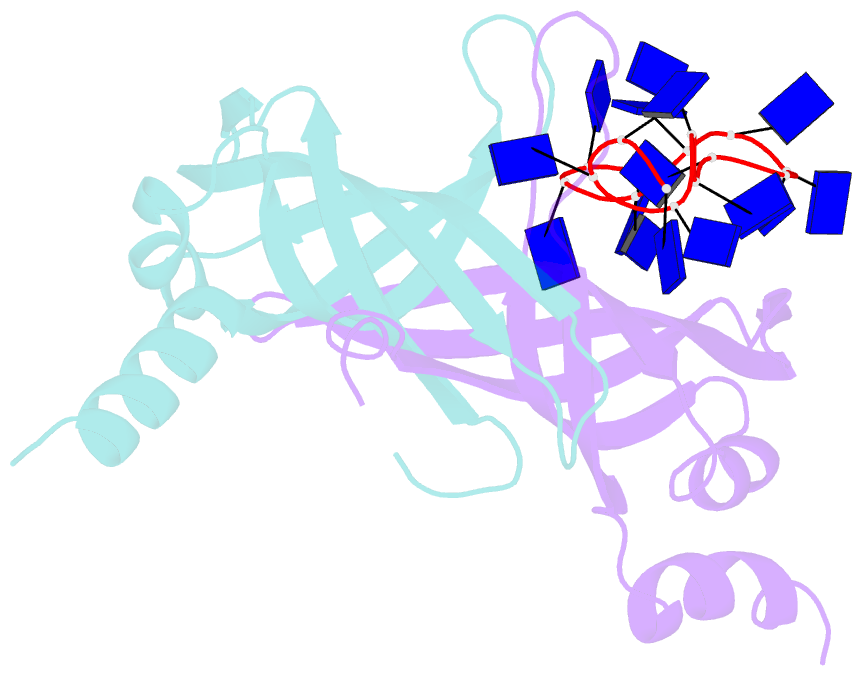
- Crystal structure of e. coli primosomol protein prib bound to ssDNA
- Reference
- Huang C-Y, Hsu C-H, Sun Y-J, Wu H-N, Hsiao C-D (2006): "Complexed Crystal Structure of Replication Restart Primsome Protein Prib Reveals a Novel Single-Stranded DNA-Binding Mode." Nucleic Acids Res., 34, 3878. doi: 10.1093/NAR/GKL536.
- Abstract
- PriB is a primosomal protein required for replication restart in Escherichia coli. PriB stimulates PriA helicase activity via interaction with single-stranded DNA (ssDNA), but the molecular details of this interaction remain unclear. Here, we report the crystal structure of PriB complexed with a 15 bases oligonucleotide (dT15) at 2.7 A resolution. PriB shares structural similarity with the E.coli ssDNA-binding protein (EcoSSB). However, the structure of the PriB-dT15 complex reveals that PriB binds ssDNA differently. Results from filter-binding assays show that PriB-ssDNA interaction is salt-sensitive and cooperative. Mutational analysis suggests that the loop L45 plays an important role in ssDNA binding. Based on the crystal structure and biochemical analyses, we propose a cooperative mechanism for the binding of PriB to ssDNA and a model for the assembly of the PriA-PriB-ssDNA complex. This report presents the first structure of a replication restart primosomal protein complexed with DNA, and a novel model that explains the interactions between a dimeric oligonucleotide-binding-fold protein and ssDNA.