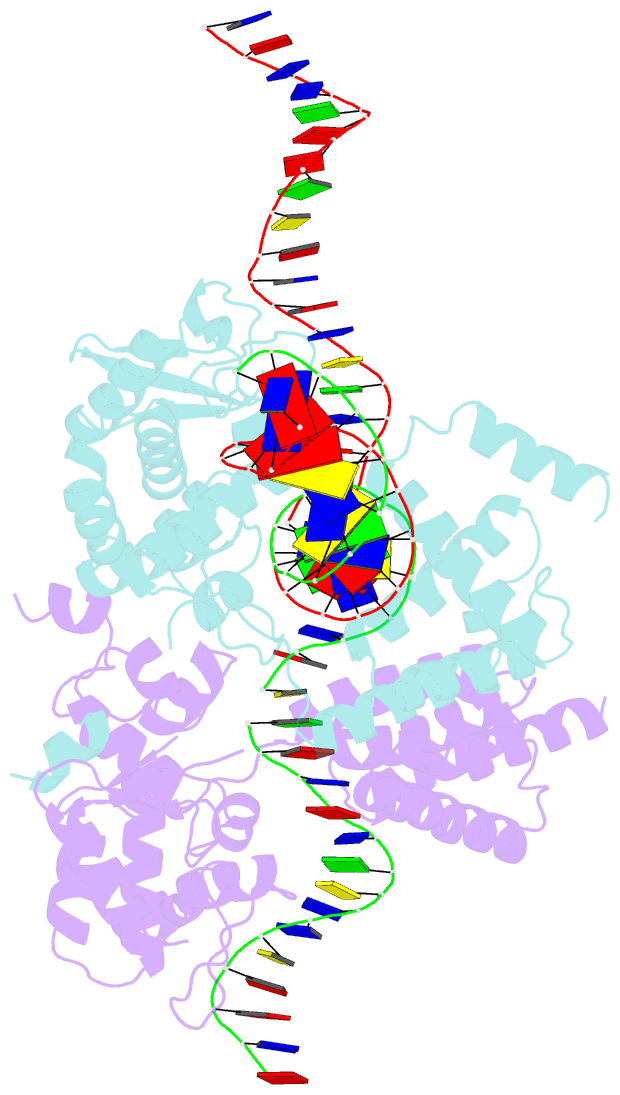
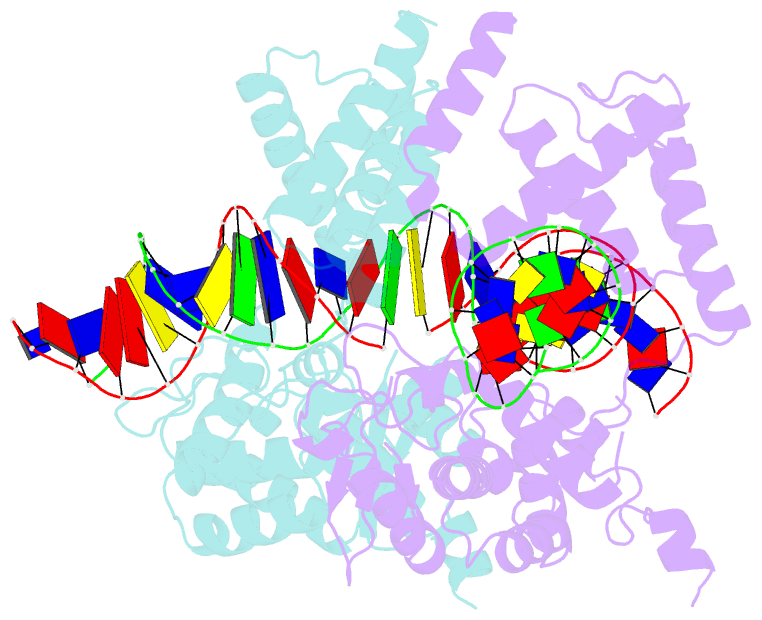
Summary information and primary citation
- PDB-id
- 2crx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase, ligase-DNA
- Method
- X-ray (2.5 Å)
- Summary
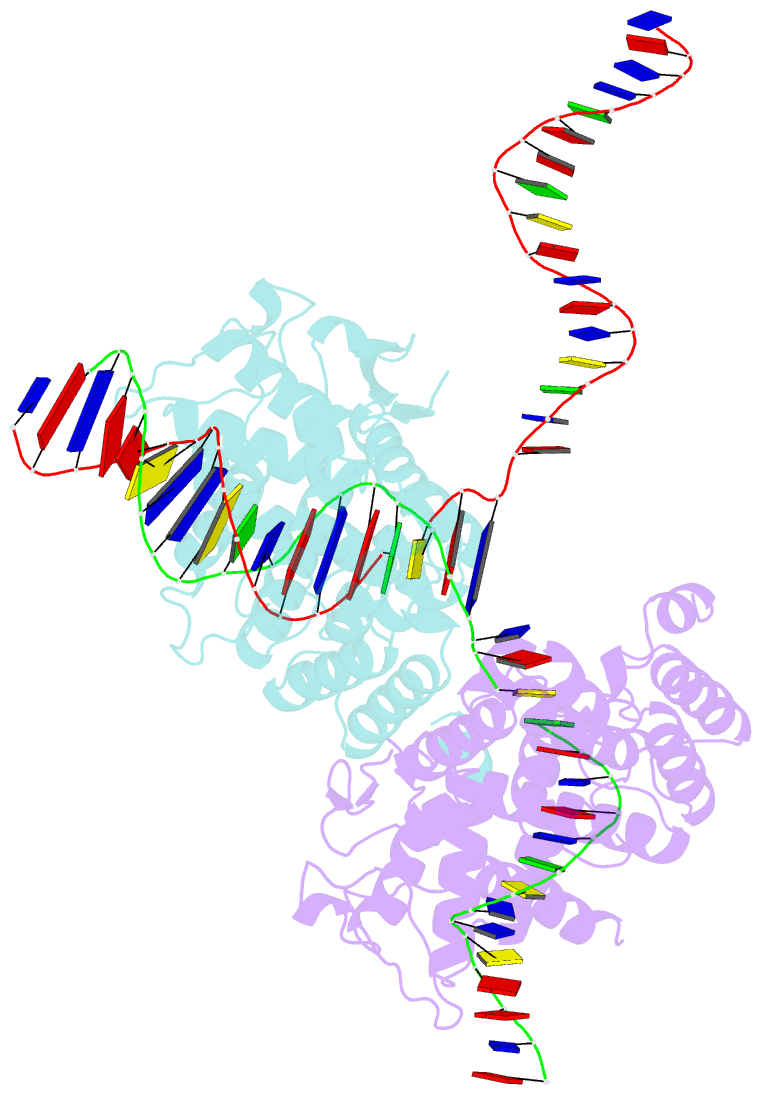
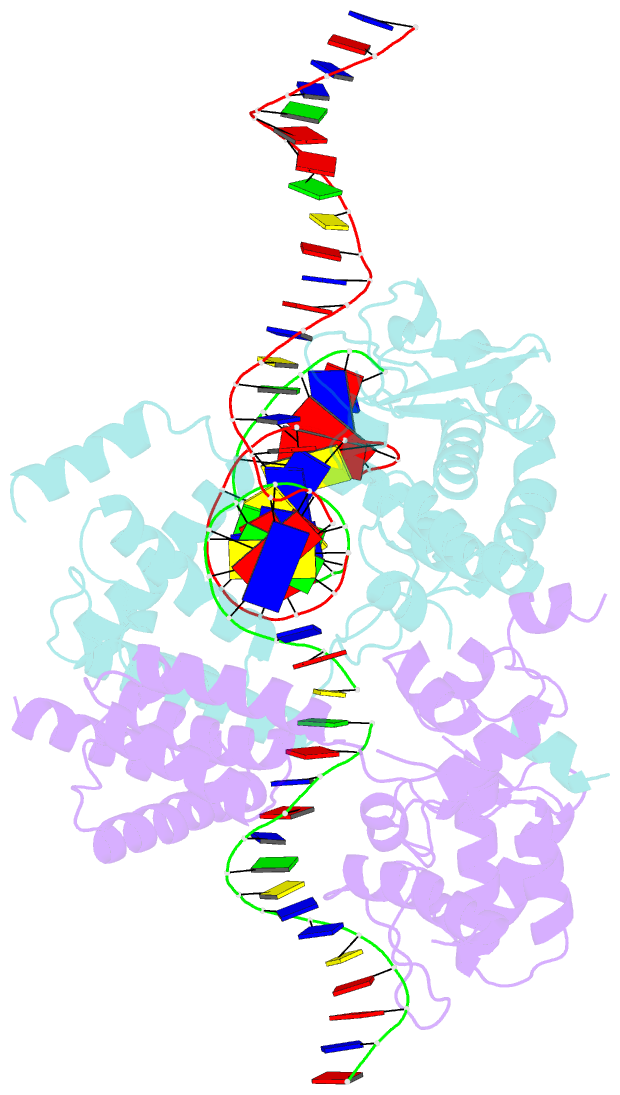
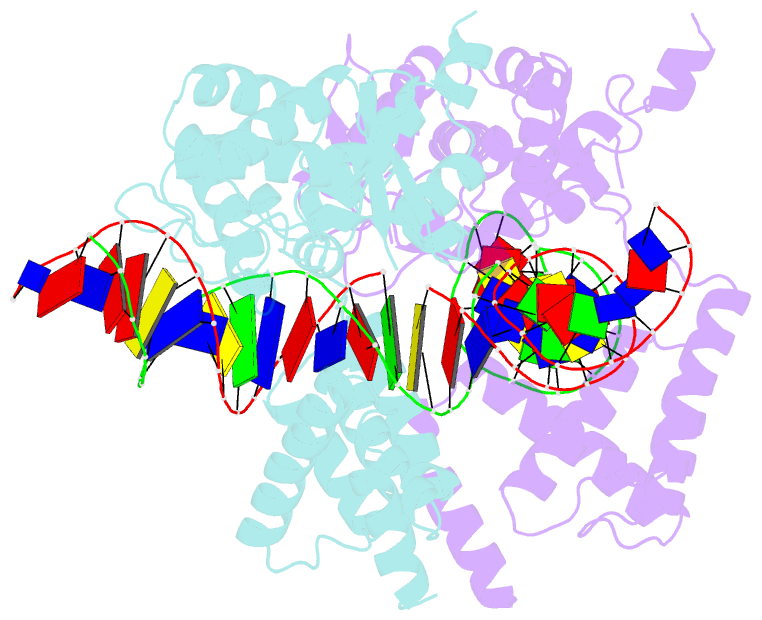
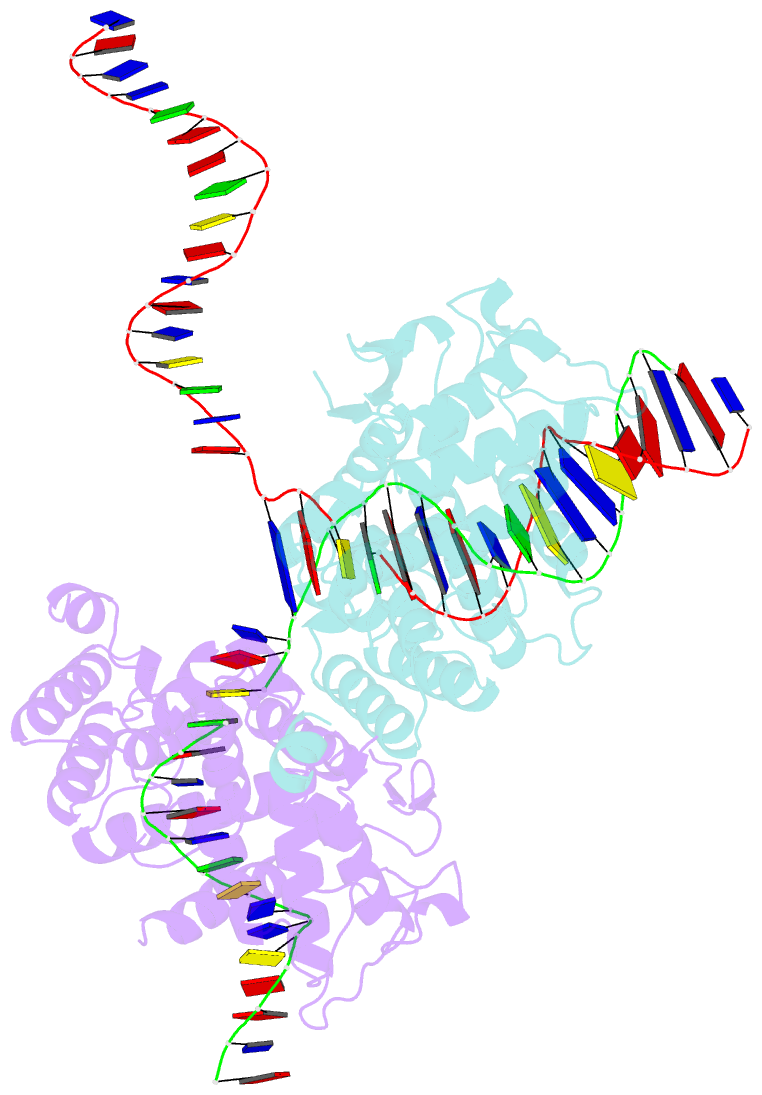
- Structure of the holliday junction intermediate in cre-loxp site-specific recombination
- Reference
- Gopaul DN, Guo F, Van Duyne GD (1998): "Structure of the Holliday junction intermediate in Cre-loxP site-specific recombination." EMBO J., 17, 4175-4187. doi: 10.1093/emboj/17.14.4175.
- Abstract
- We have determined the X-ray crystal structures of two DNA Holliday junctions (HJs) bound by Cre recombinase. The HJ is a four-way branched structure that occurs as an intermediate in genetic recombination pathways, including site-specific recombination by the lambda-integrase family. Cre recombinase is an integrase family member that recombines 34 bp loxP sites in the absence of accessory proteins or auxiliary DNA sequences. The 2.7 A structure of Cre recombinase bound to an immobile HJ and the 2.5 A structure of Cre recombinase bound to a symmetric, nicked HJ reveal a nearly planar, twofold-symmetric DNA intermediate that shares features with both the stacked-X and the square conformations of the HJ that exist in the unbound state. The structures support a protein-mediated crossover isomerization of the junction that acts as the switch responsible for activation and deactivation of recombinase active sites. In this model, a subtle isomerization of the Cre recombinase-HJ quaternary structure dictates which strands are cleaved during resolution of the junction via a mechanism that involves neither branch migration nor helical restacking.