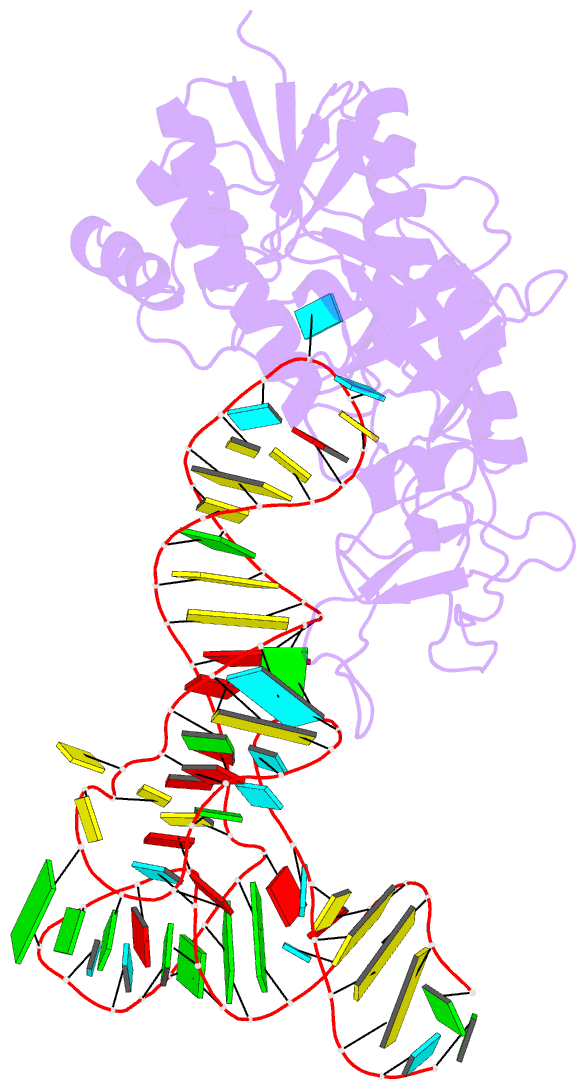
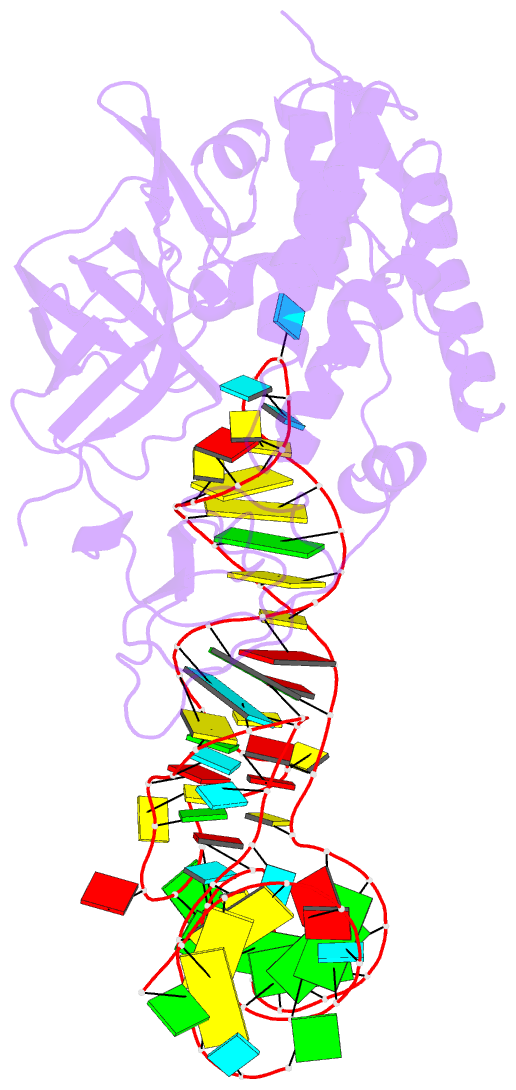
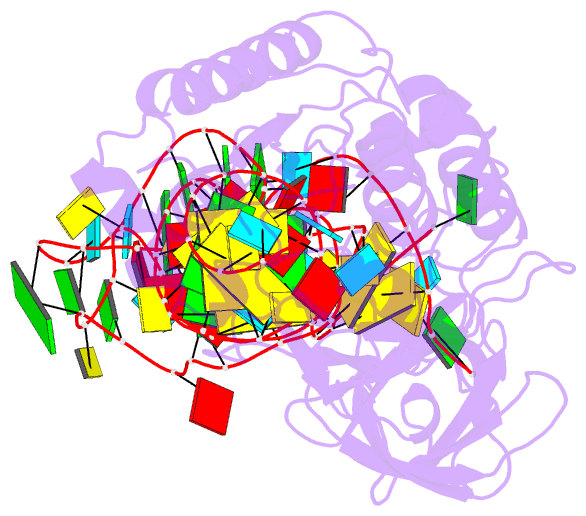
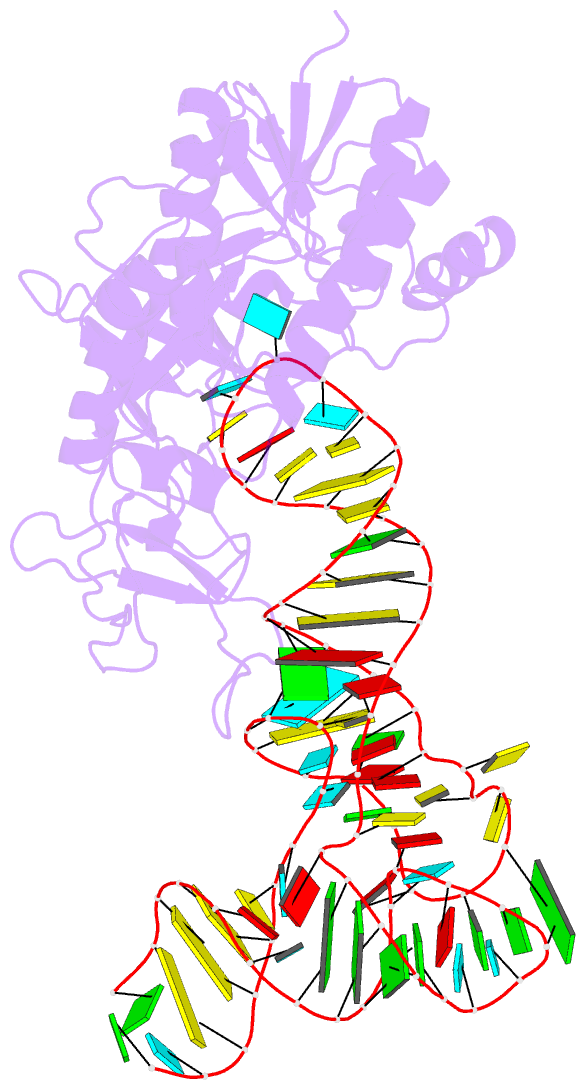
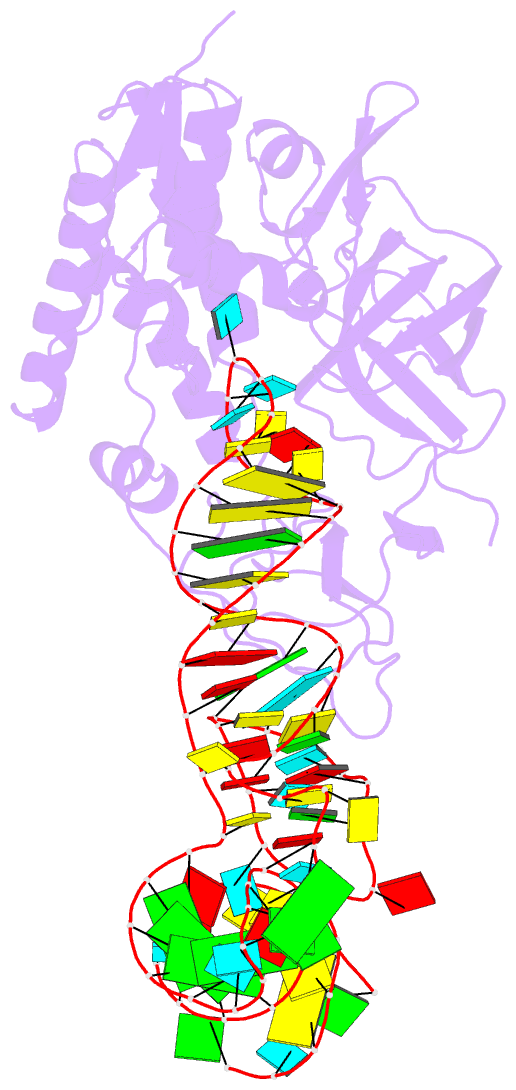
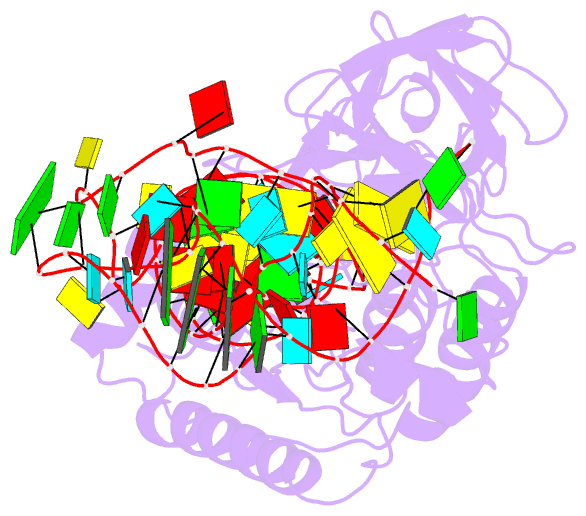
Summary information and primary citation
- PDB-id
- 2det; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (3.4 Å)
- Summary
- Cocrystal structure of an RNA sulfuration enzyme mnma and trna-glu in the pre-reaction state
- Reference
- Numata T, Ikeuchi Y, Fukai S, Suzuki T, Nureki O (2006): "Snapshots of tRNA sulphuration via an adenylated intermediate." Nature, 442, 419-424. doi: 10.1038/nature04896.
- Abstract
- Uridine at the first anticodon position (U34) of glutamate, lysine and glutamine transfer RNAs is universally modified by thiouridylase into 2-thiouridine (s2U34), which is crucial for precise translation by restricting codon-anticodon wobble during protein synthesis on the ribosome. However, it remains unclear how the enzyme incorporates reactive sulphur into the correct position of the uridine base. Here we present the crystal structures of the MnmA thiouridylase-tRNA complex in three discrete forms, which provide snapshots of the sequential chemical reactions during RNA sulphuration. On enzyme activation, an alpha-helix overhanging the active site is restructured into an idiosyncratic beta-hairpin-containing loop, which packs the flipped-out U34 deeply into the catalytic pocket and triggers the activation of the catalytic cysteine residues. The adenylated RNA intermediate is trapped. Thus, the active closed-conformation of the complex ensures accurate sulphur incorporation into the activated uridine carbon by forming a catalytic chamber to prevent solvent from accessing the catalytic site. The structures of the complex with glutamate tRNA further reveal how MnmA specifically recognizes its three different tRNA substrates. These findings provide the structural basis for a general mechanism whereby an enzyme incorporates a reactive atom at a precise position in a biological molecule.