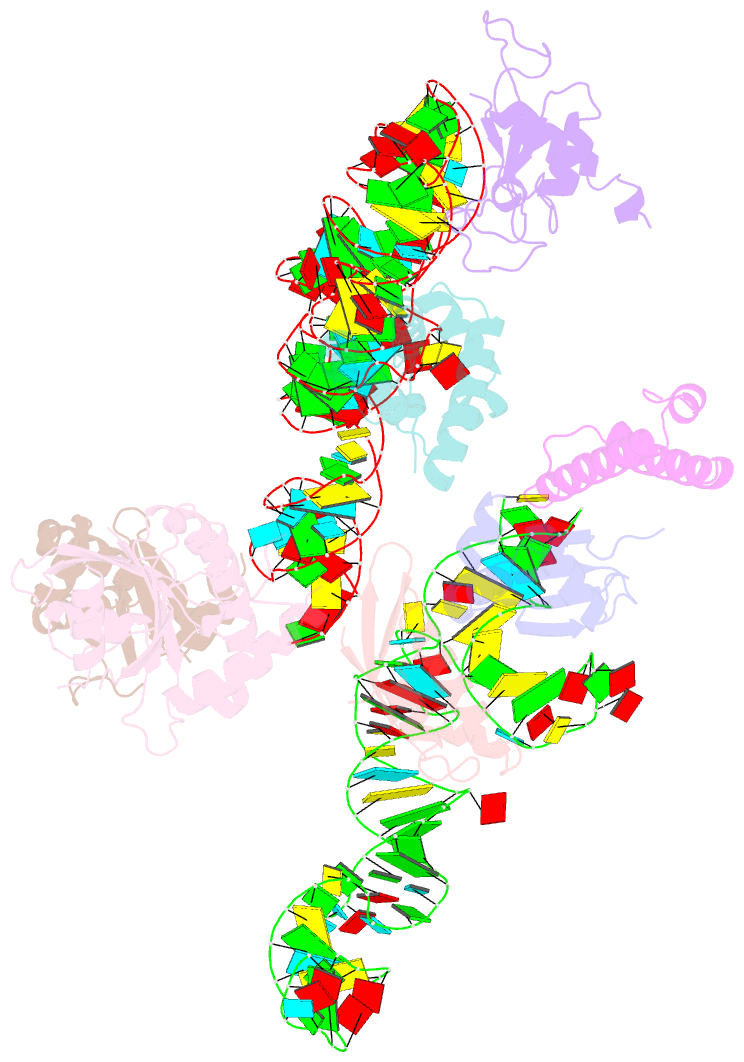
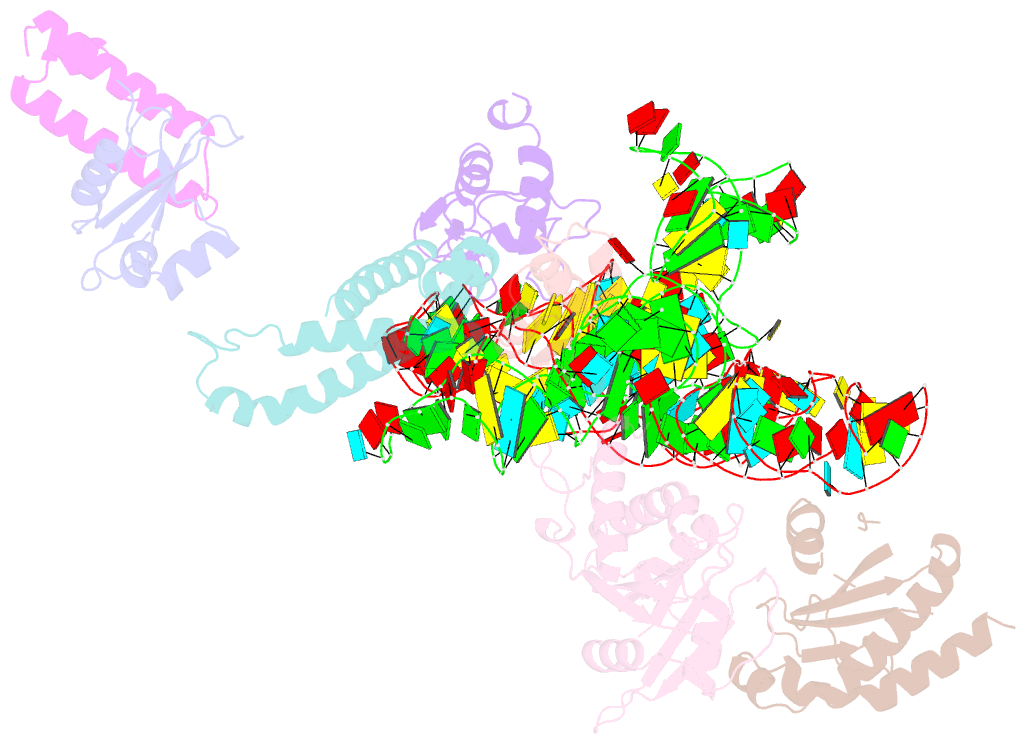
Summary information and primary citation
- PDB-id
- 2go5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation-RNA
- Method
- cryo-EM (7.4 Å)
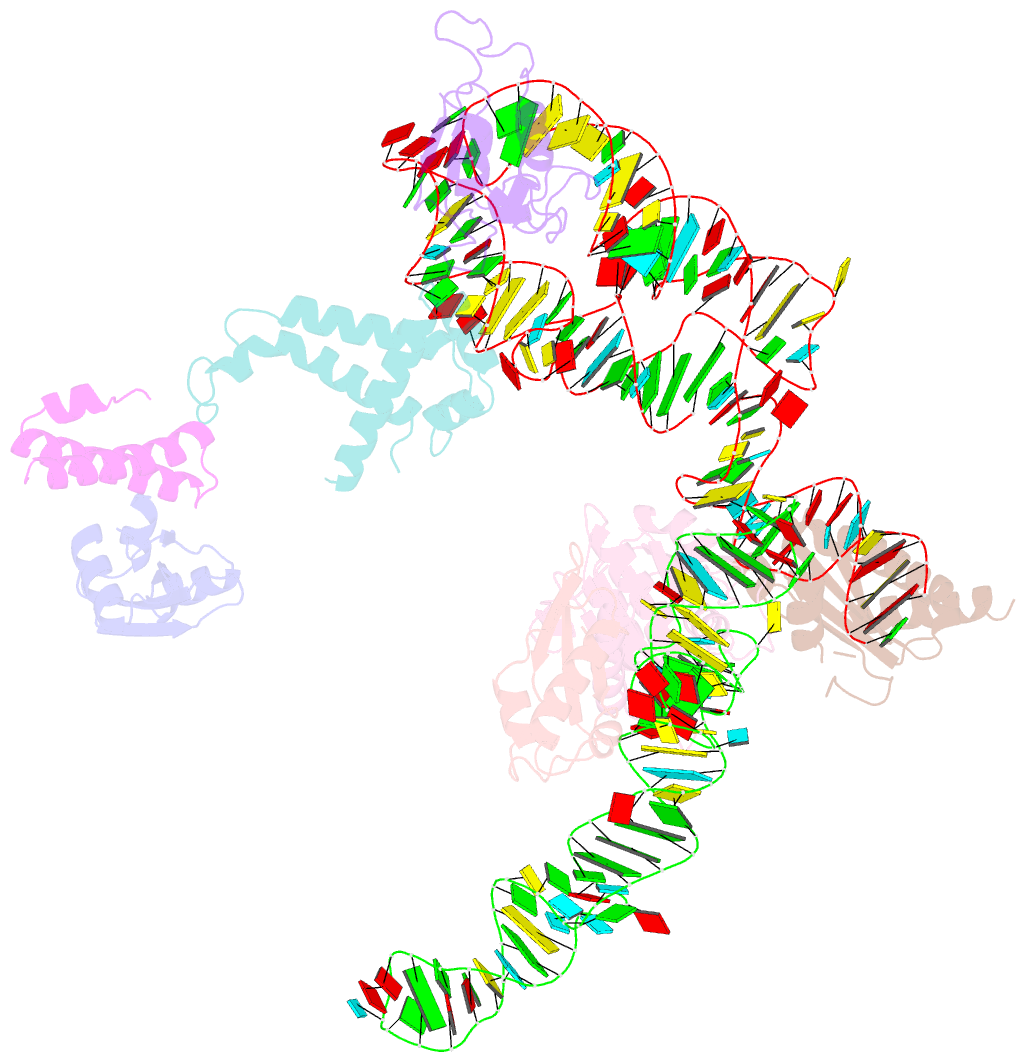
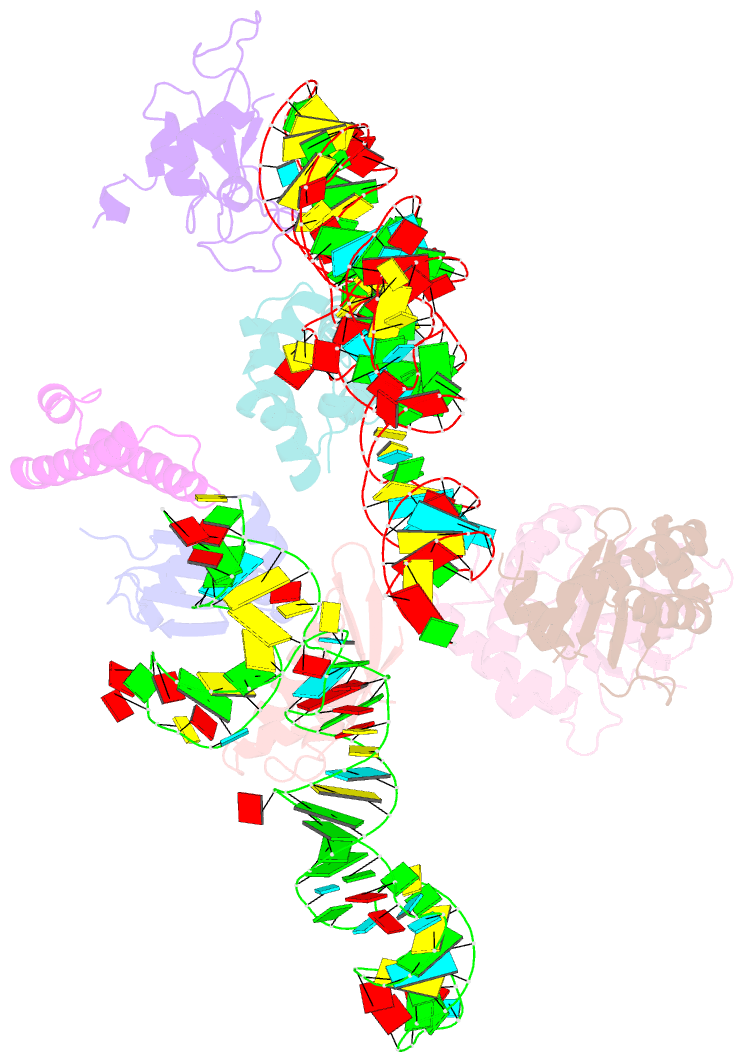
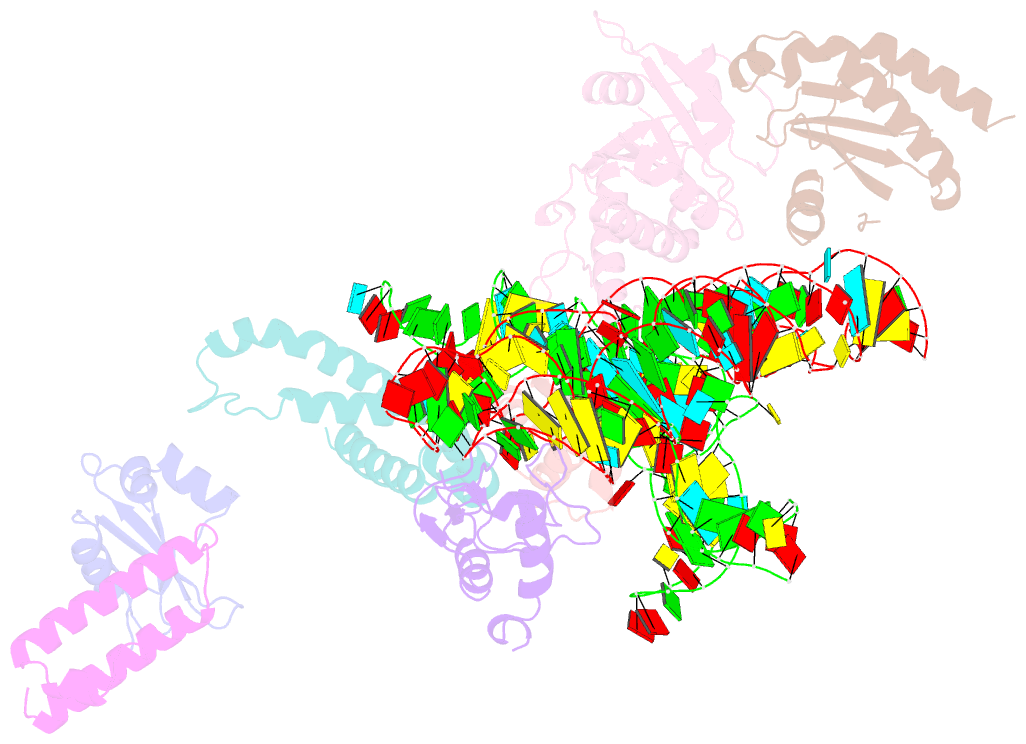
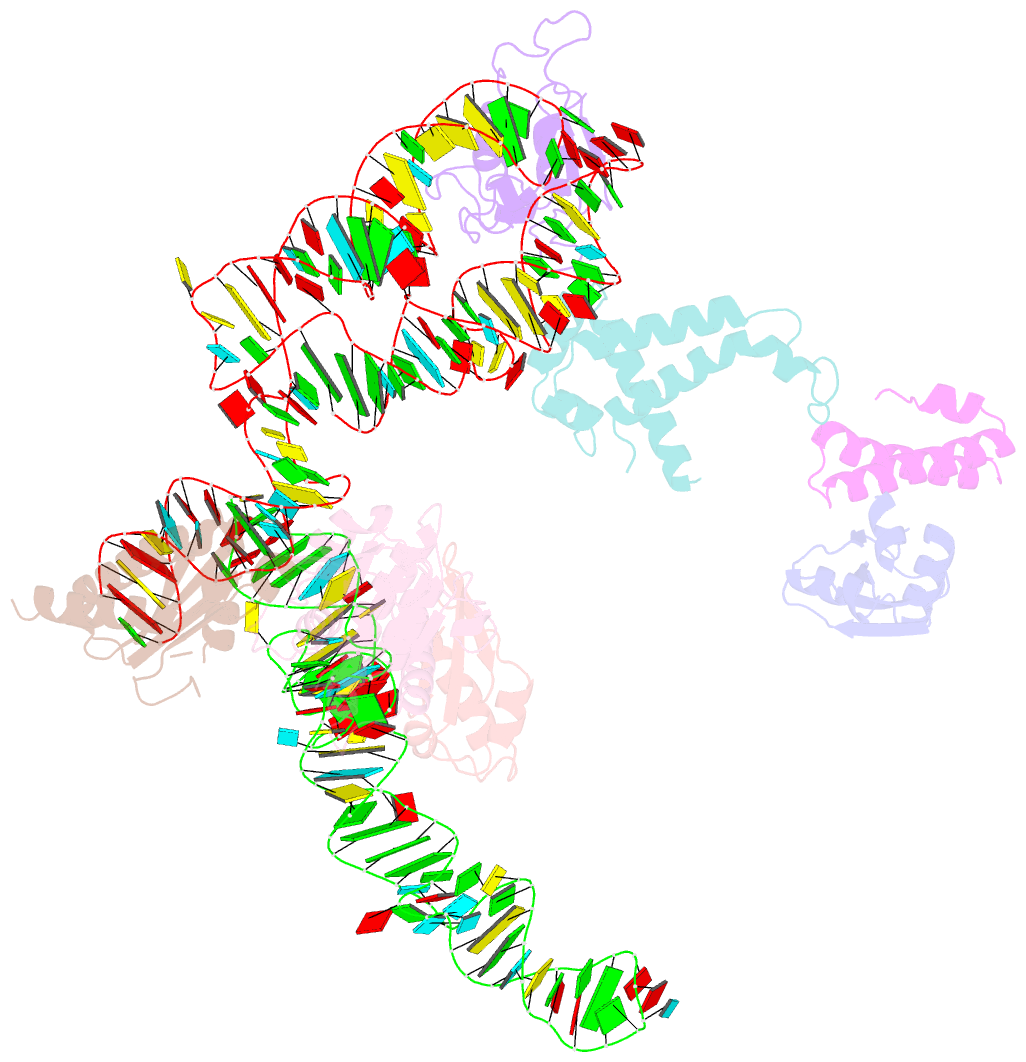
- Summary
- Structure of signal recognition particle receptor (sr) in complex with signal recognition particle (srp) and ribosome nascent chain complex
- Reference
- Halic M, Gartmann M, Schlenker O, Mielke T, Pool MR, Sinning I, Beckmann R (2006): "Signal Recognition Particle Receptor Exposes the Ribosomal Translocon Binding Site." Science, 312, 745-747. doi: 10.1126/science.1124864.
- Abstract
- Signal sequences of secretory and membrane proteins are recognized by the signal recognition particle (SRP) as they emerge from the ribosome. This results in their targeting to the membrane by docking with the SRP receptor, which facilitates transfer of the ribosome to the translocon. Here, we present the 8 angstrom cryo-electron microscopy structure of a "docking complex" consisting of a SRP-bound 80S ribosome and the SRP receptor. Interaction of the SRP receptor with both SRP and the ribosome rearranged the S domain of SRP such that a ribosomal binding site for the translocon, the L23e/L35 site, became exposed, whereas Alu domain-mediated elongation arrest persisted.