Summary information and primary citation
- PDB-id
- 2gxb; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.25 Å)
- Summary
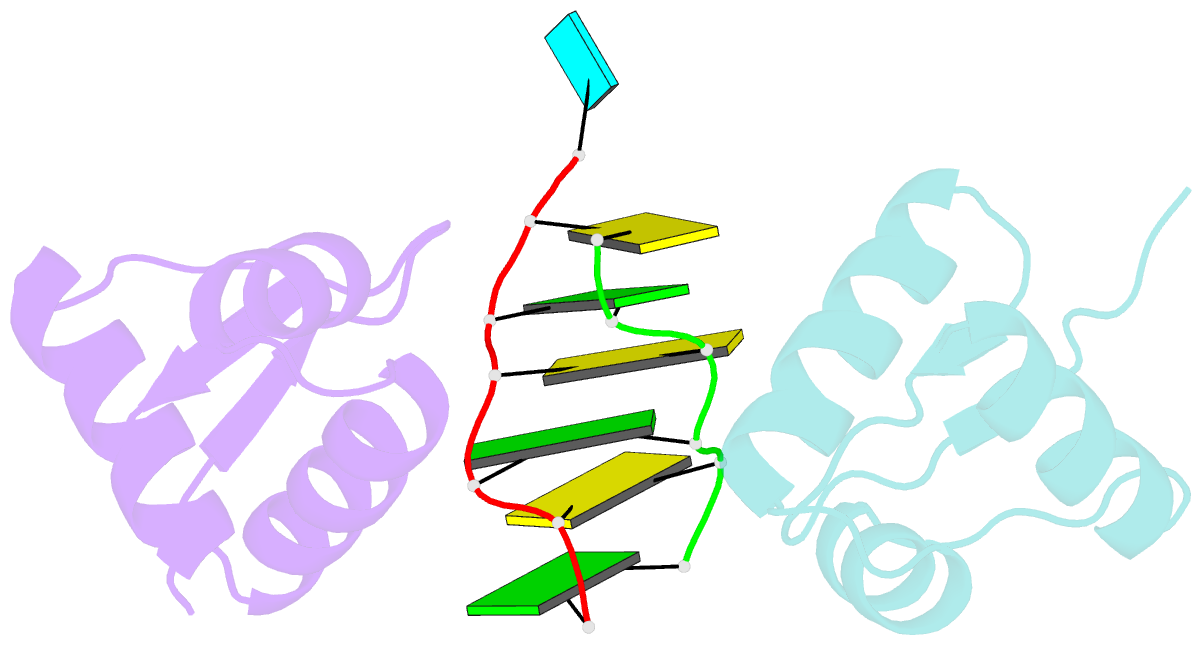
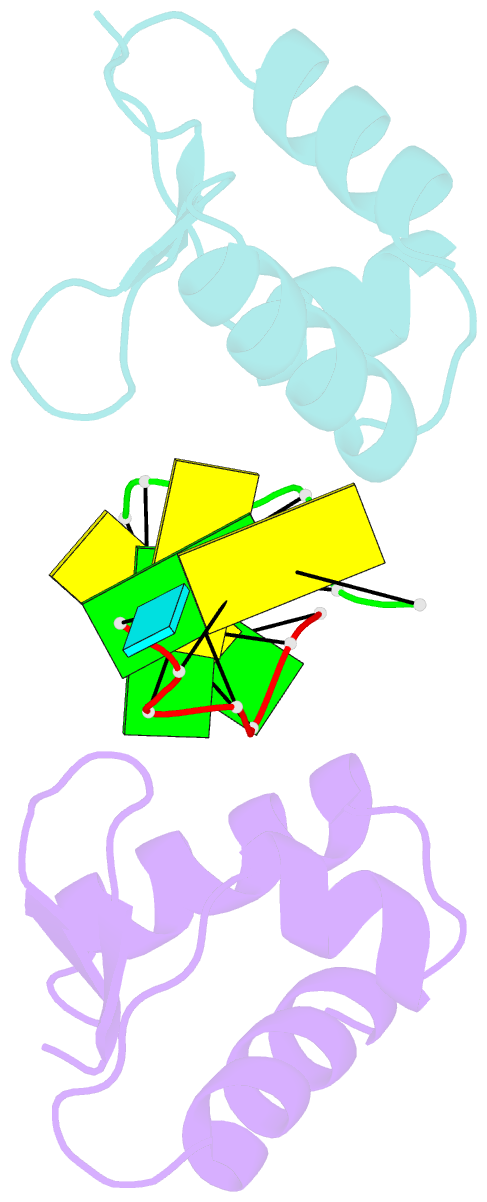
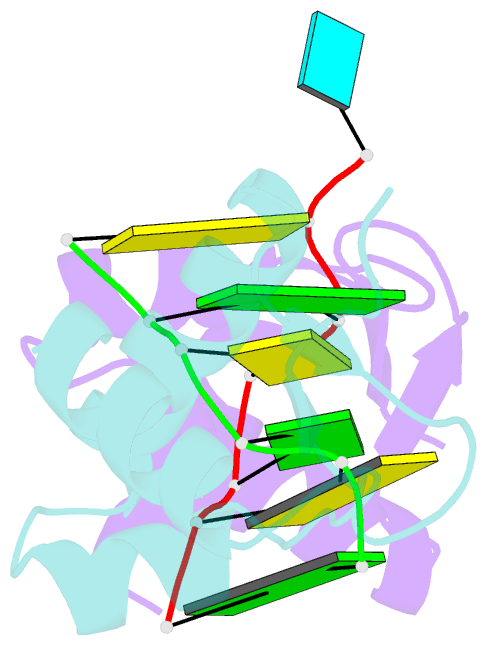
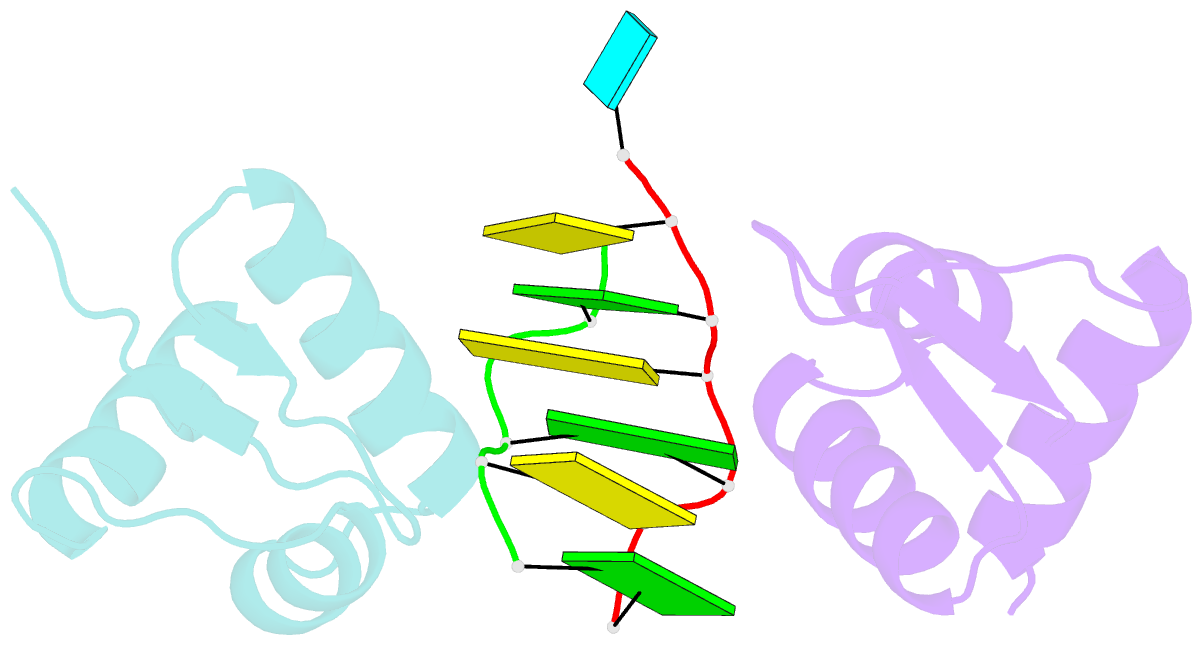
- Crystal structure of the za domain bound to z-RNA
- Reference
- Placido D, Brown BA, Lowenhaupt K, Rich A, Athanasiadis A (2007): "A Left-Handed RNA Double Helix Bound by the Zalpha Domain of the RNA-Editing Enzyme ADAR1." Structure, 15, 395-404. doi: 10.1016/j.str.2007.03.001.
- Abstract
- The A form RNA double helix can be transformed to a left-handed helix, called Z-RNA. Currently, little is known about the detailed structural features of Z-RNA or its involvement in cellular processes. The discovery that certain interferon-response proteins have domains that can stabilize Z-RNA as well as Z-DNA opens the way for the study of Z-RNA. Here, we present the 2.25 A crystal structure of the Zalpha domain of the RNA-editing enzyme ADAR1 (double-stranded RNA adenosine deaminase) complexed to a dUr(CG)(3) duplex RNA. The Z-RNA helix is associated with a unique solvent pattern that distinguishes it from the otherwise similar conformation of Z-DNA. Based on the structure, we propose a model suggesting how differences in solvation lead to two types of Z-RNA structures. The interaction of Zalpha with Z-RNA demonstrates how the interferon-induced isoform of ADAR1 could be targeted toward selected dsRNAs containing purine-pyrimidine repeats, possibly of viral origin.