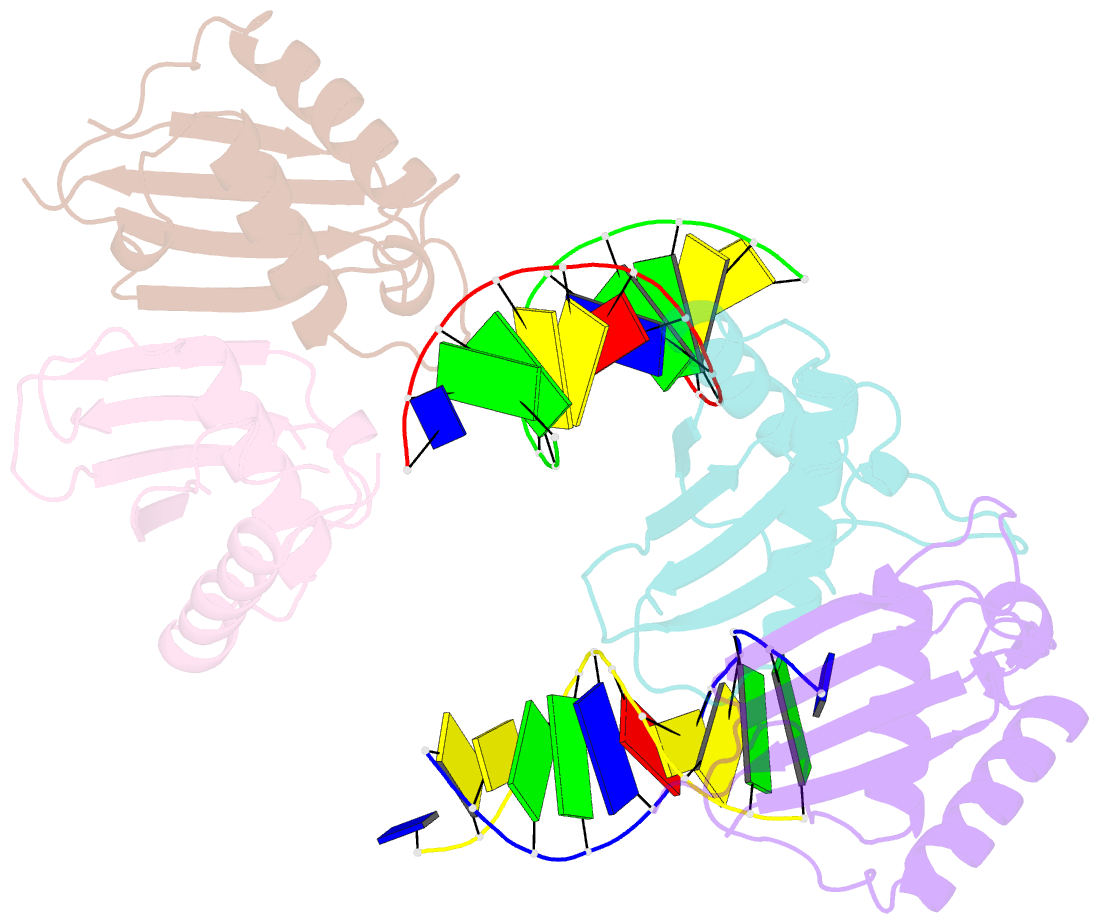
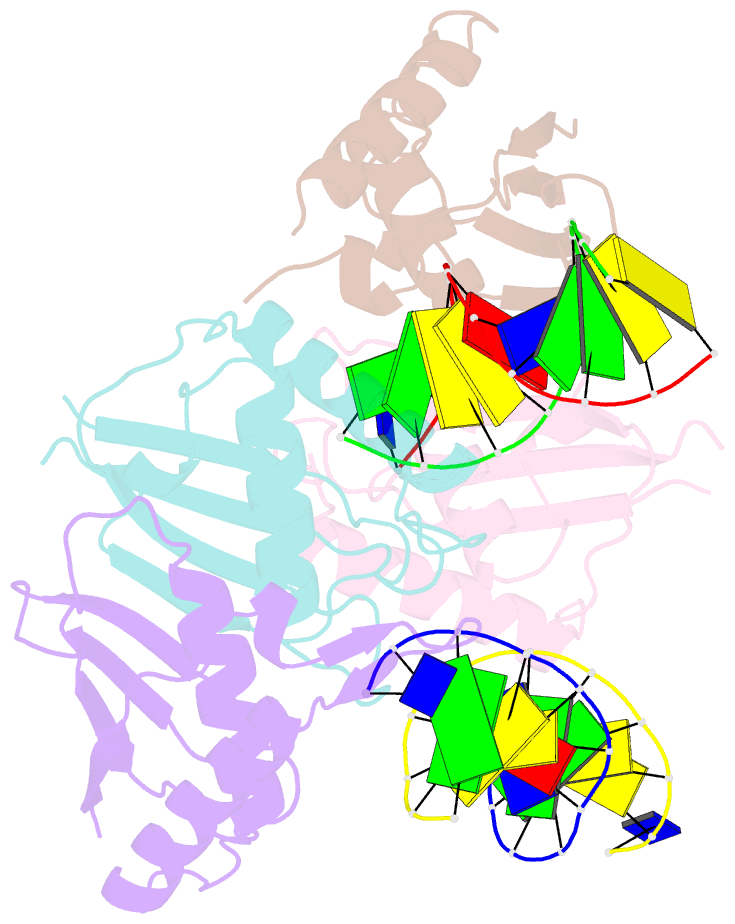
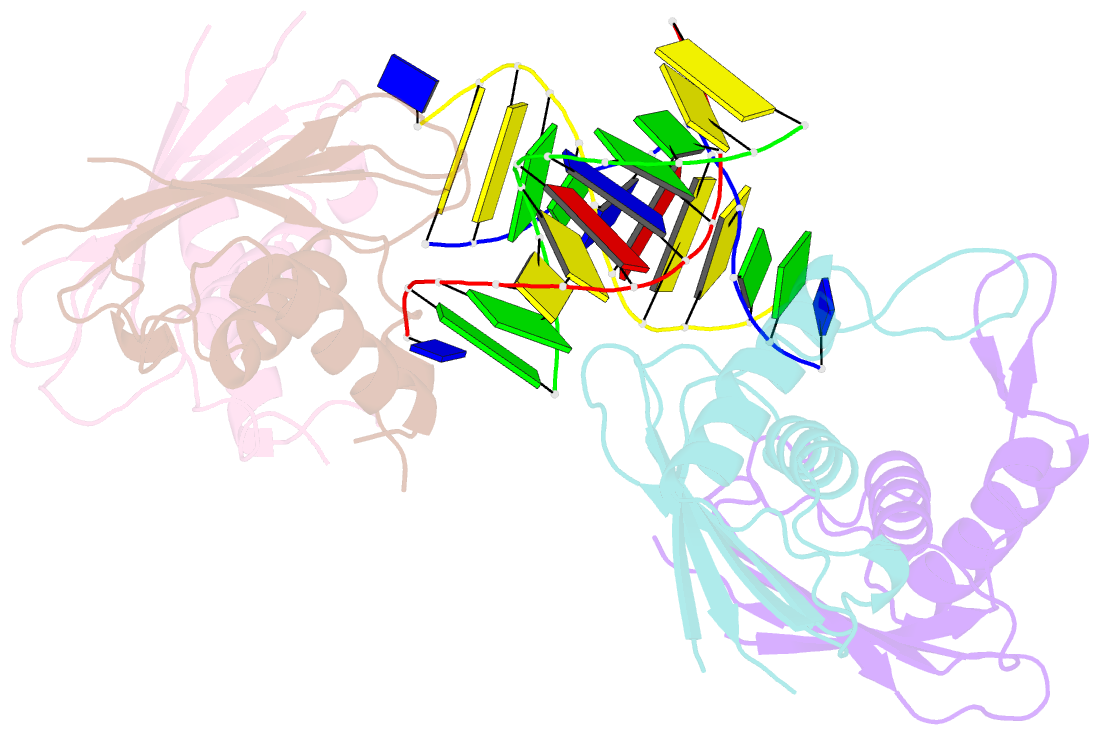
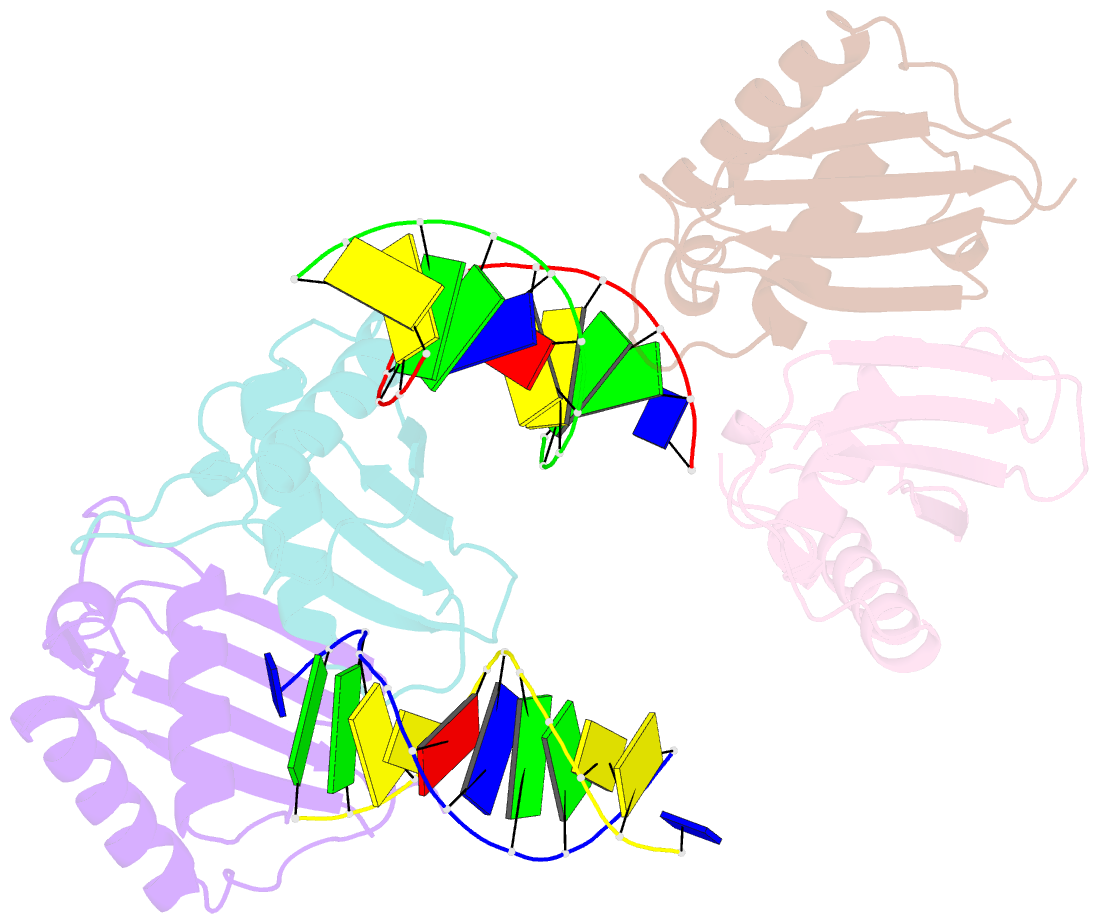
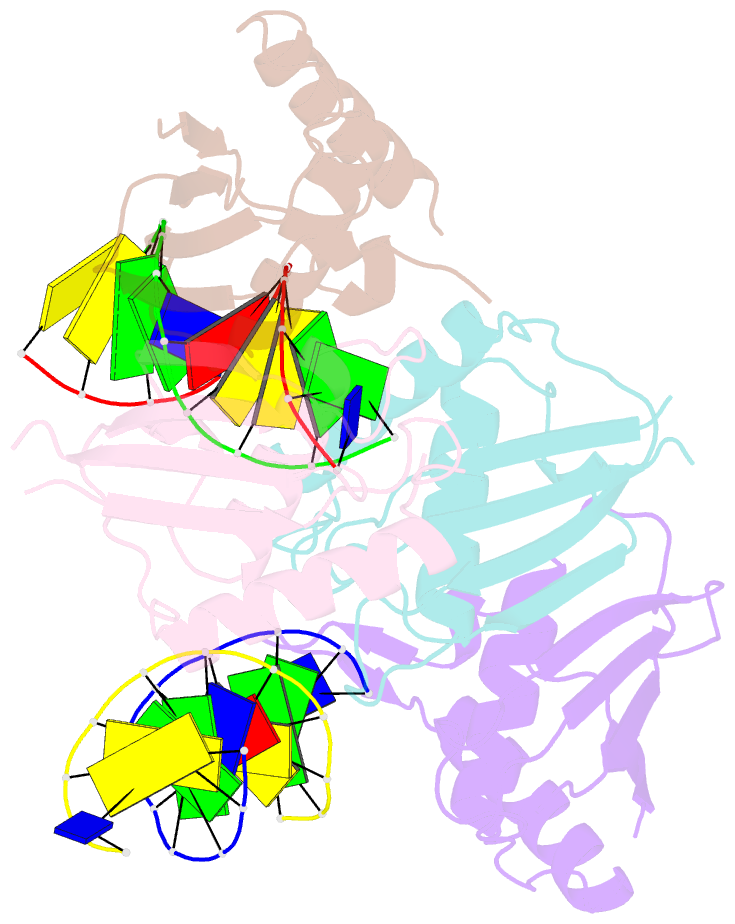
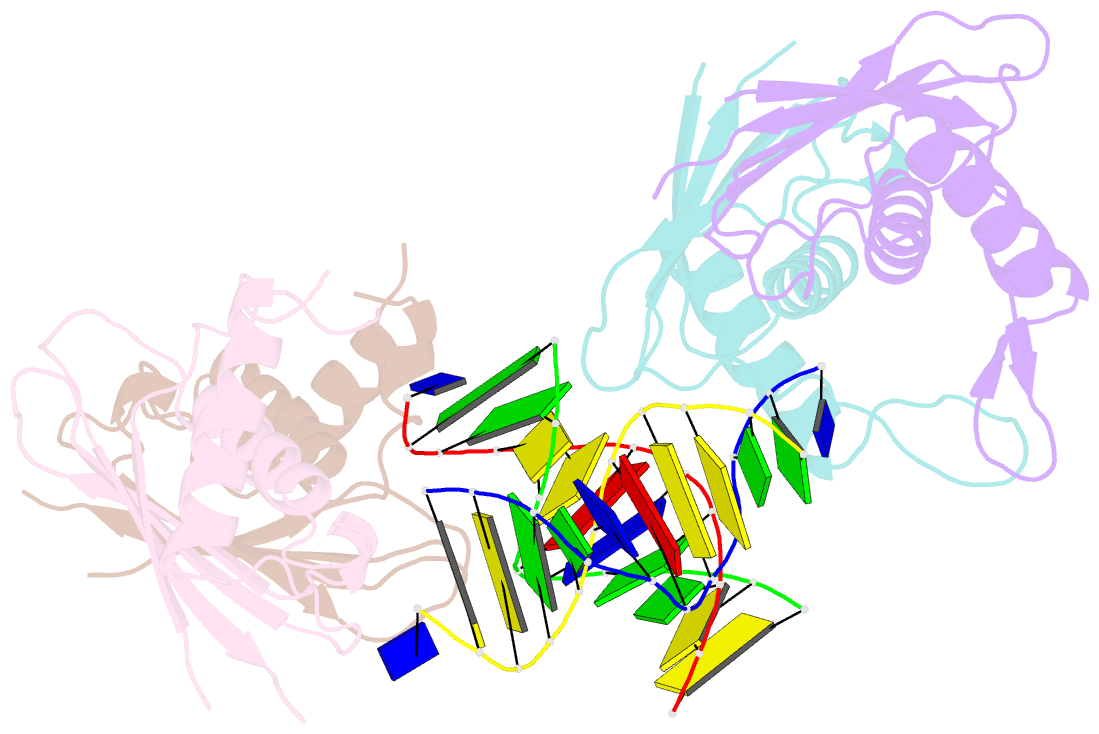
Summary information and primary citation
- PDB-id
- 2h8c; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.1 Å)
- Summary
- Structure of rusa d70n in complex with DNA
- Reference
- Macmaster R, Sedelnikova S, Baker PJ, Bolt EL, Lloyd RG, Rafferty JB (2006): "RusA Holliday junction resolvase: DNA complex structure--insights into selectivity and specificity." Nucleic Acids Res., 34, 5577-5584. doi: 10.1093/nar/gkl447.
- Abstract
- We have determined the structure of a catalytically inactive D70N variant of the Escherichia coli RusA resolvase bound to a duplex DNA substrate that reveals critical protein-DNA interactions and permits a much clearer understanding of the interaction of the enzyme with a Holliday junction (HJ). The RusA enzyme cleaves HJs, the fourway DNA branchpoints formed by homologous recombination, by introducing symmetrical cuts in the phosphodiester backbone in a Mg2+ dependent reaction. Although, RusA shows a high level of selectivity for DNA junctions, preferring to bind fourway junctions over other substrates in vitro, it has also been shown to have appreciable affinity for duplex DNA. However, RusA does not show DNA cleavage activity with duplex substrates. Our structure suggests the possible basis for structural selectivity as well as sources of the sequence specificity observed for DNA cleavage by RusA.