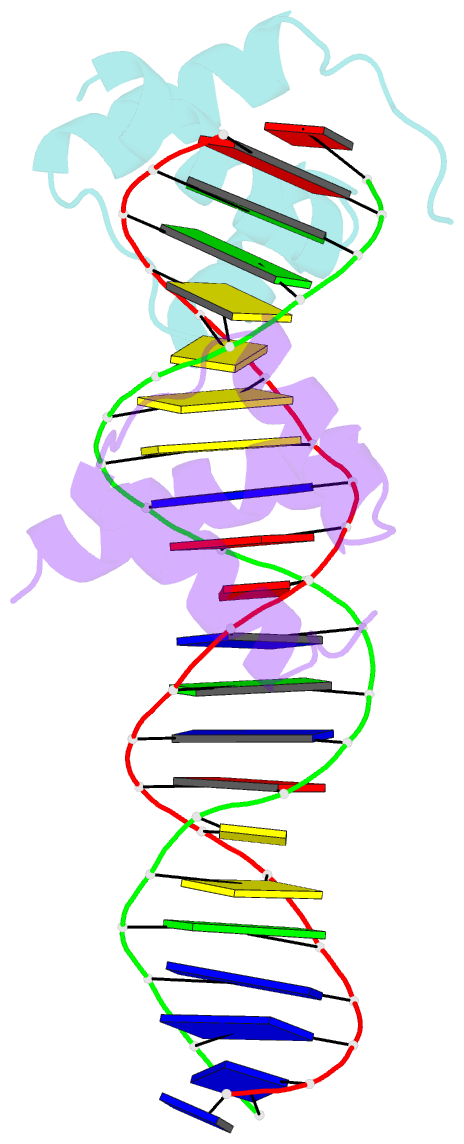
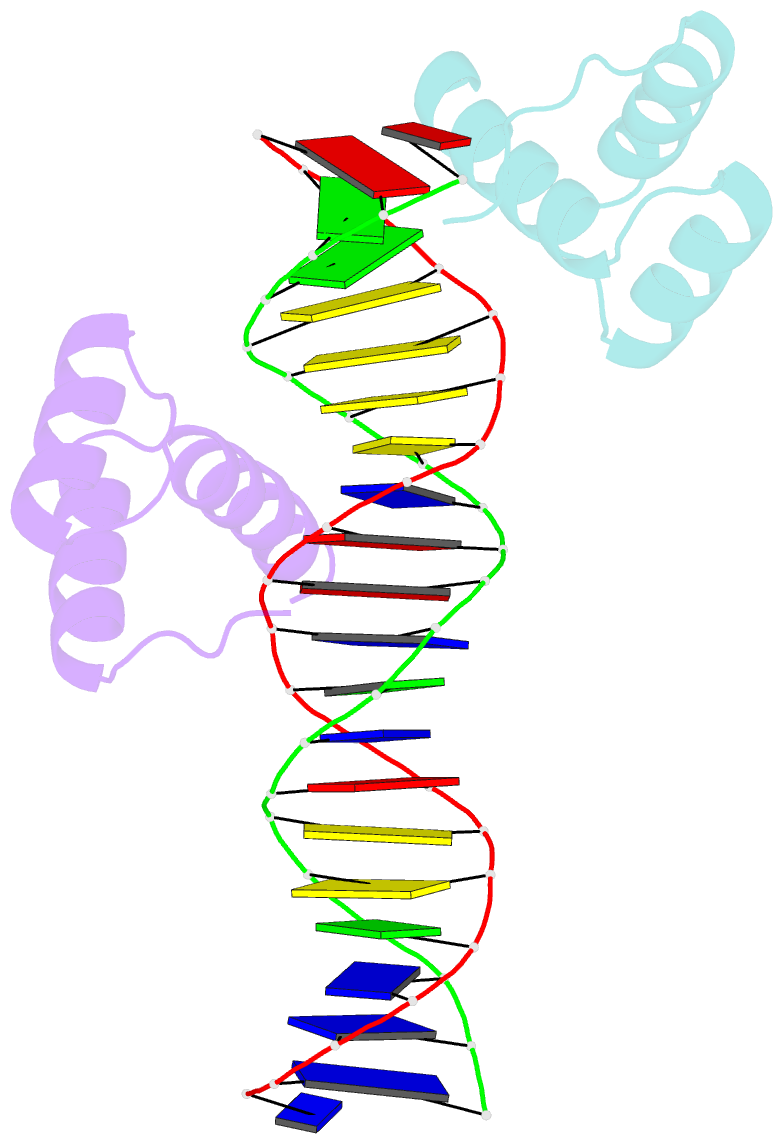
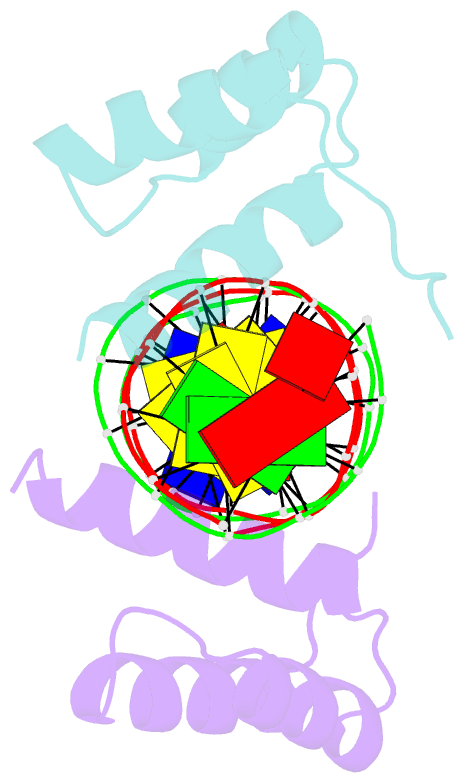
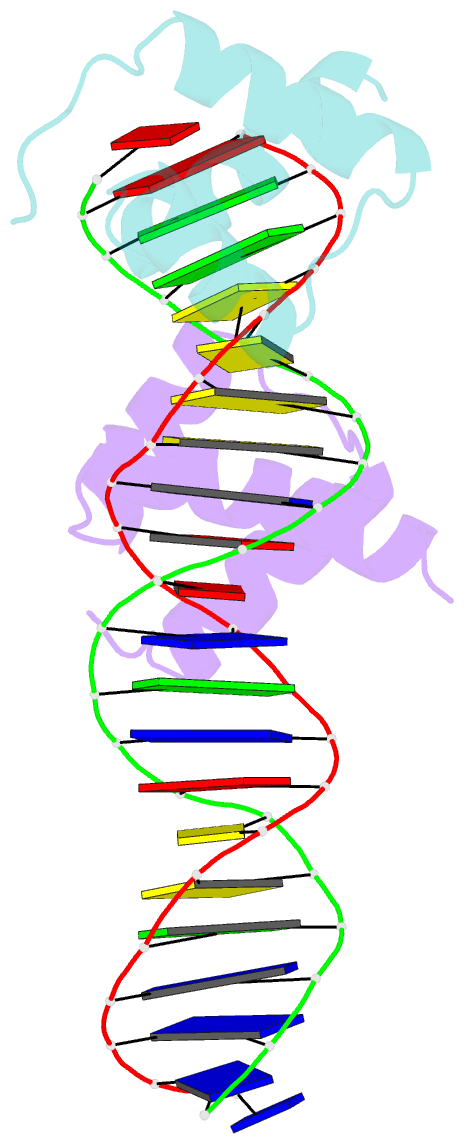
Summary information and primary citation
- PDB-id
- 2hdd; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (1.9 Å)
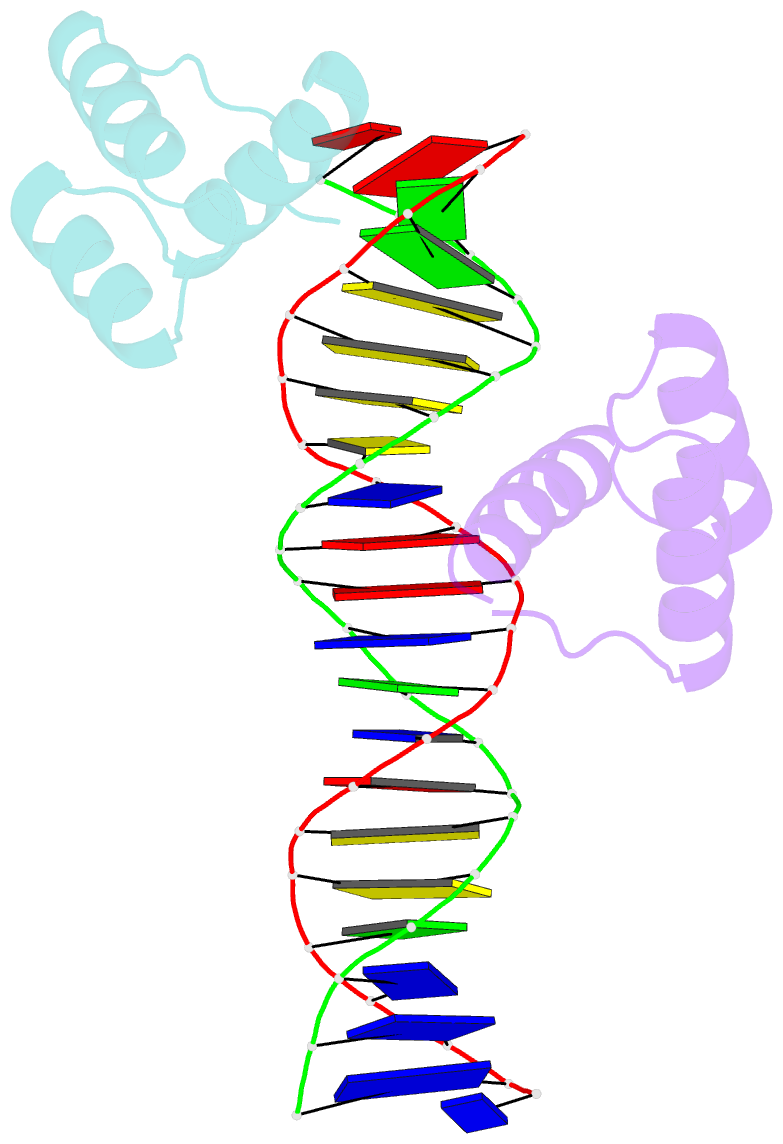
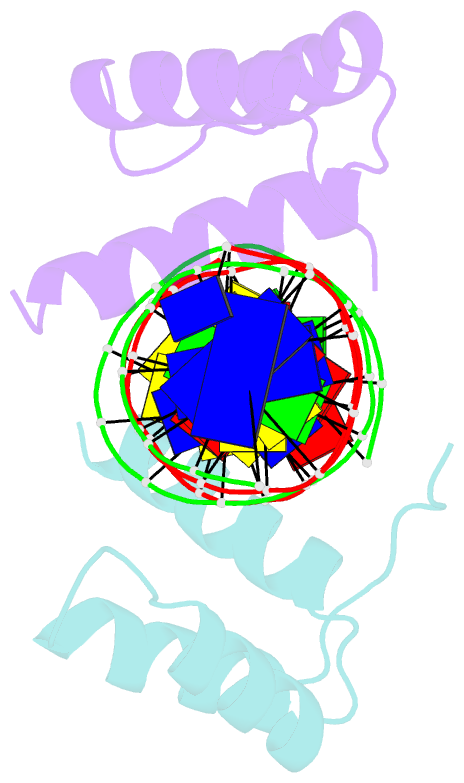
- Summary
- Engrailed homeodomain q50k variant DNA complex
- Reference
- Tucker-Kellogg L, Rould MA, Chambers KA, Ades SE, Sauer RT, Pabo CO (1997): "Engrailed (Gln50-->Lys) homeodomain-DNA complex at 1.9 A resolution: structural basis for enhanced affinity and altered specificity." Structure, 5, 1047-1054. doi: 10.1016/S0969-2126(97)00256-6.
- Abstract
- Background: The homeodomain is one of the key DNA-binding motifs used in eukaryotic gene regulation, and homeodomain proteins play critical roles in development. The residue at position 50 of many homeodomains appears to determine the differential DNA-binding specificity, helping to distinguish among binding sites of the form TAATNN. However, the precise role(s) of residue 50 in the differential recognition of alternative sites has not been clear. None of the previously determined structures of homeodomain-DNA complexes has shown evidence for a stable hydrogen bond between residue 50 and a base, and there has been much discussion, based in part on NMR studies, about the potential importance of water-mediated contacts. This study was initiated to help clarify some of these issues.
Results: The crystal structure of a complex containing the engrailed Gln50-->Lys variant (QK50) with its optimal binding site TAATCC (versus TAATTA for the wild-type protein) has been determined at 1.9 A resolution. The overall structure of the QK50 variant is very similar to that of the wild-type complex, but the sidechain of Lys50 projects directly into the major groove and makes several hydrogen bonds to the O6 and N7 atoms of the guanines at base pairs 5 and 6. Lys50 also makes an additional water-mediated contact with the guanine at base pair 5 and has an alternative conformation that allows a hydrogen bond with the O4 of the thymine at base pair 4.
Conclusions: The structural context provided by the folding and docking of the engrailed homeodomain allows Lys50 to make remarkably favorable contacts with the guanines at base pairs 5 and 6 of the binding site. Although many different residues occur at position 50 in different homeodomains, and although numerous position 50 variants have been constructed, the most striking examples of altered specificity usually involve introducing or removing a lysine sidechain from position 50. This high-resolution structure also confirms the critical role of Asn51 in homeodomain-DNA recognition and further clarifies the roles of water molecules near residues 50 and 51.