Summary information and primary citation
- PDB-id
- 2hhh; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (3.35 Å)
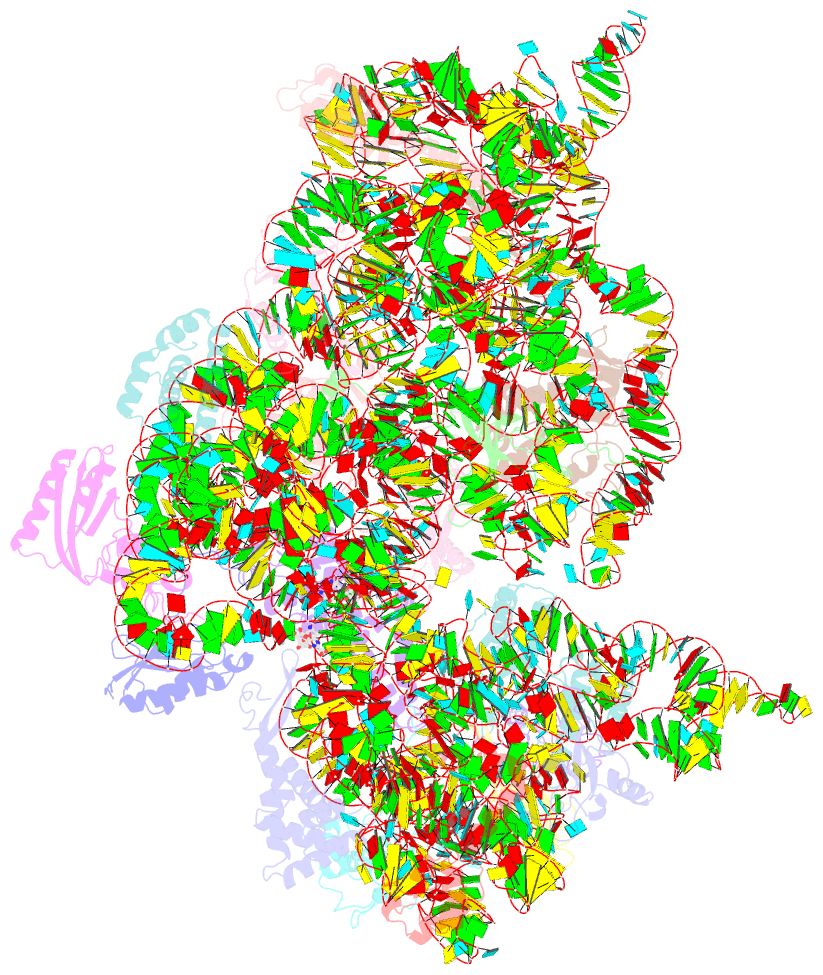
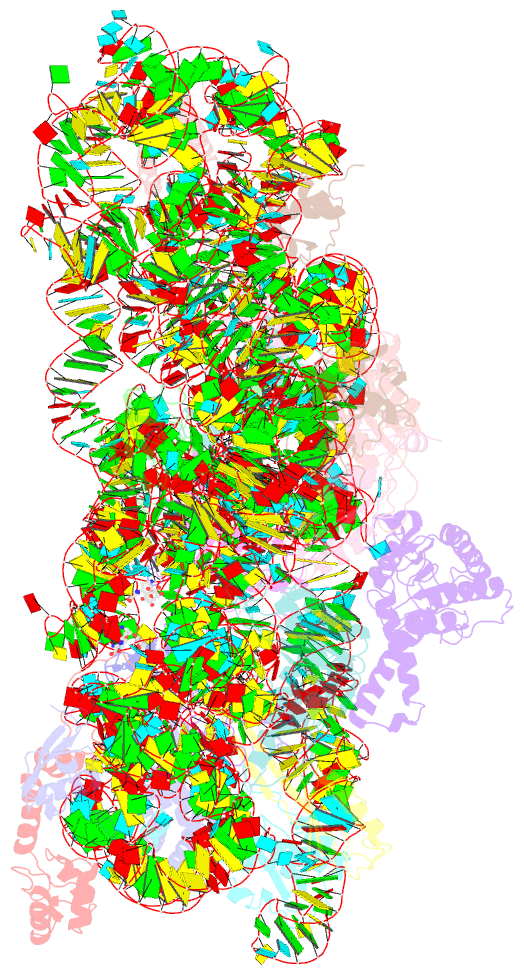
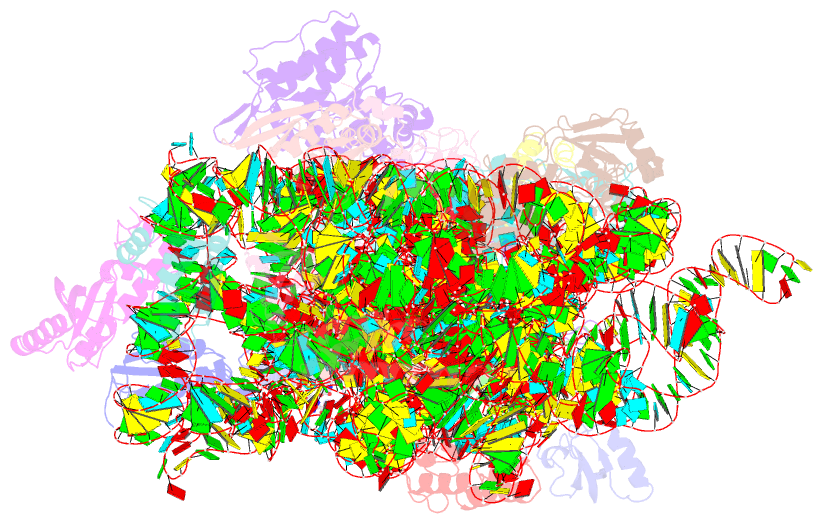
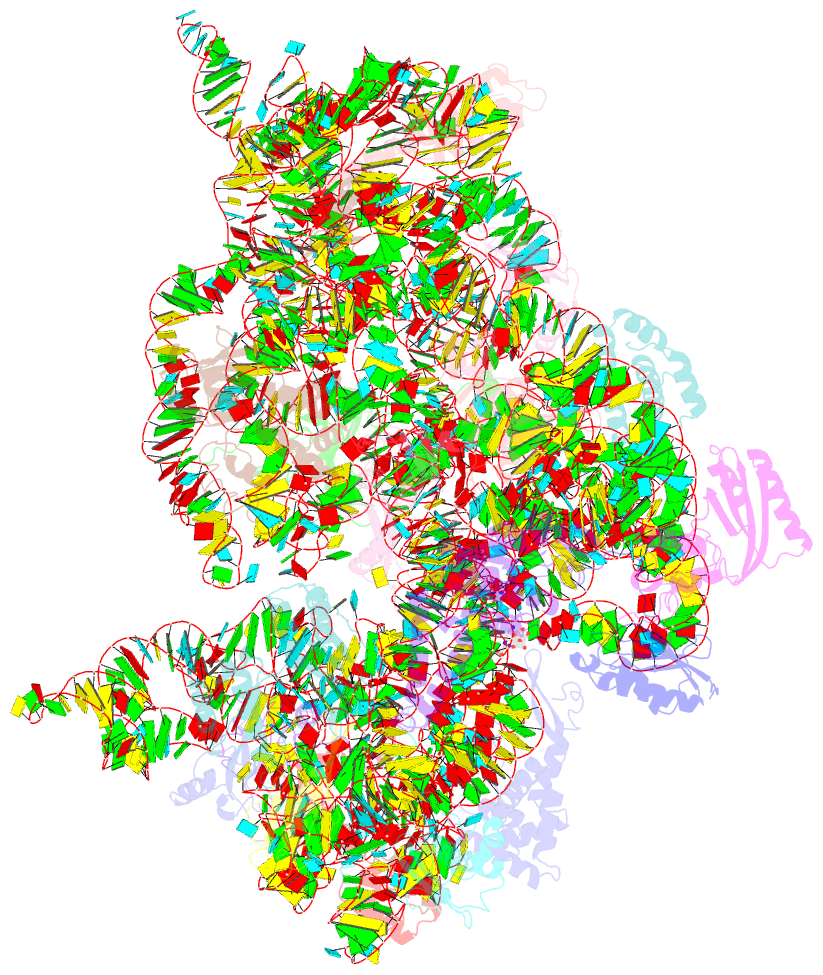
- Summary
- Crystal structure of kasugamycin bound to the 30s ribosomal subunit
- Reference
- Schluenzen F, Takemoto C, Wilson DN, Kaminishi T, Harms JM, Hanawa-Suetsugu K, Szaflarski W, Kawazoe M, Shirouzo M, Nierhaus KH, Yokoyama S, Fucini P (2006): "The antibiotic kasugamycin mimics mRNA nucleotides to destabilize tRNA binding and inhibit canonical translation initiation." Nat.Struct.Mol.Biol., 13, 871-878. doi: 10.1038/nsmb1145.
- Abstract
- Kasugamycin (Ksg) specifically inhibits translation initiation of canonical but not of leaderless messenger RNAs. Ksg inhibition is thought to occur by direct competition with initiator transfer RNA. The 3.35-A structure of Ksg bound to the 30S ribosomal subunit presented here provides a structural description of two Ksg-binding sites as well as a basis for understanding Ksg resistance. Notably, neither binding position overlaps with P-site tRNA; instead, Ksg mimics codon nucleotides at the P and E sites by binding within the path of the mRNA. Coupled with biochemical experiments, our results suggest that Ksg indirectly inhibits P-site tRNA binding through perturbation of the mRNA-tRNA codon-anticodon interaction during 30S canonical initiation. In contrast, for 70S-type initiation on leaderless mRNA, the overlap between mRNA and Ksg is reduced and the binding of tRNA is further stabilized by the presence of the 50S subunit, minimizing Ksg efficacy.