Summary information and primary citation
- PDB-id
- 2hvs; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-DNA-RNA
- Method
- X-ray (2.5 Å)
- Summary
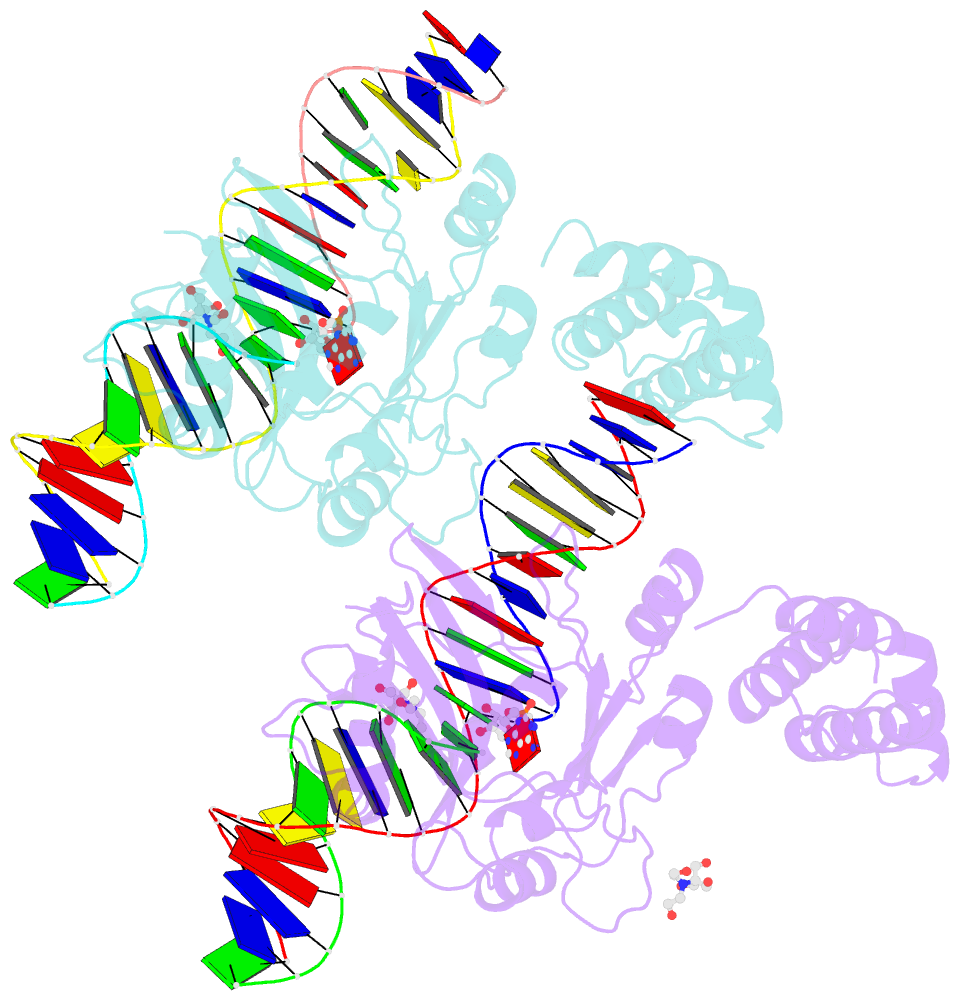
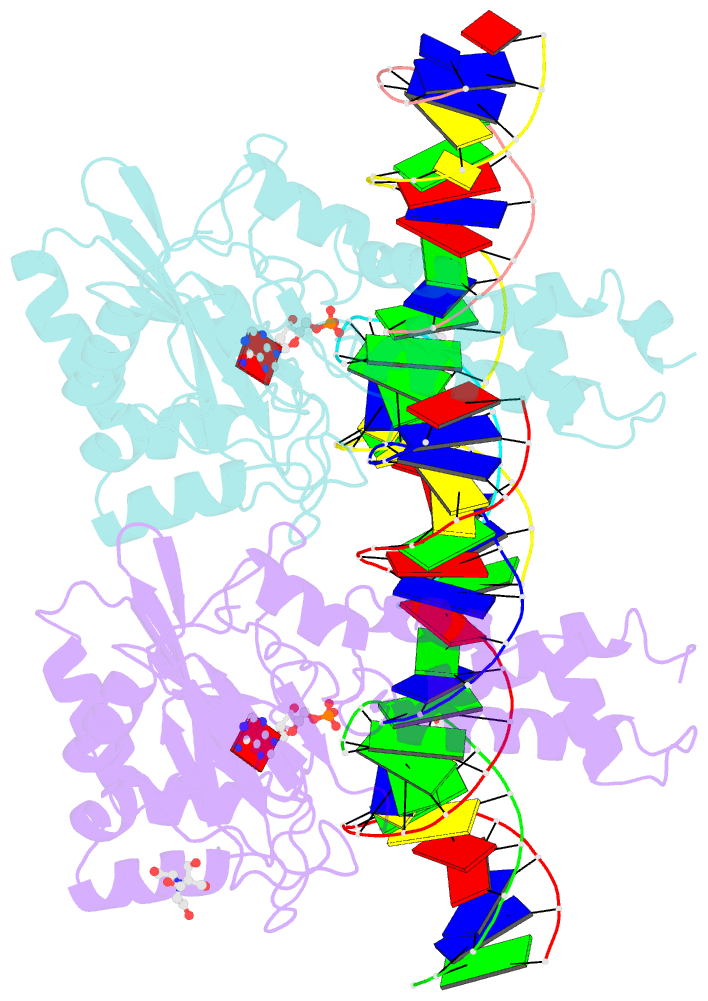
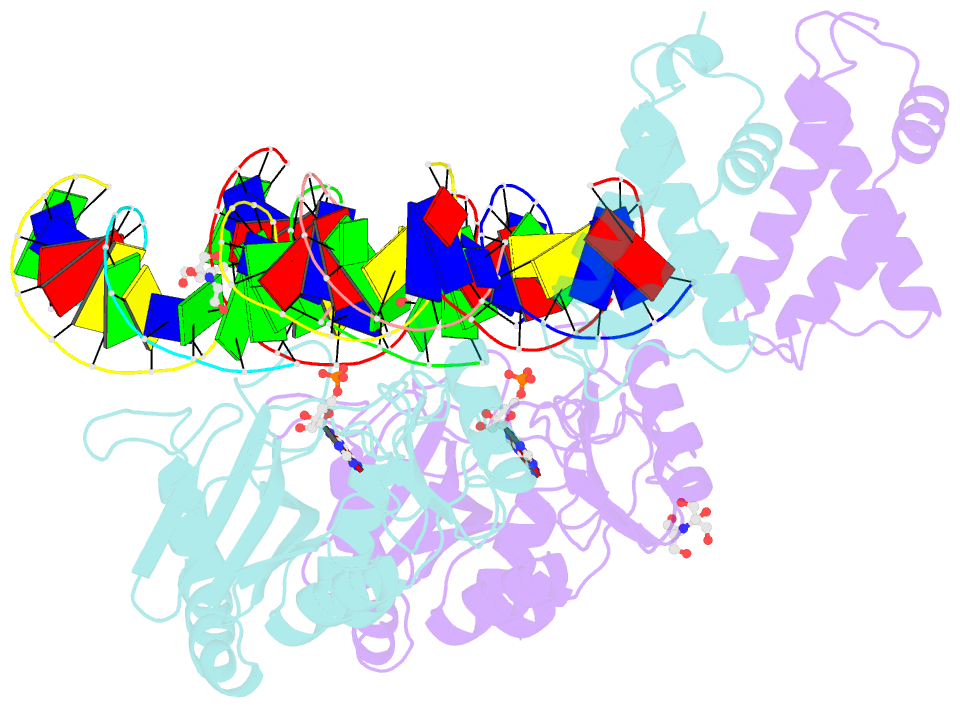
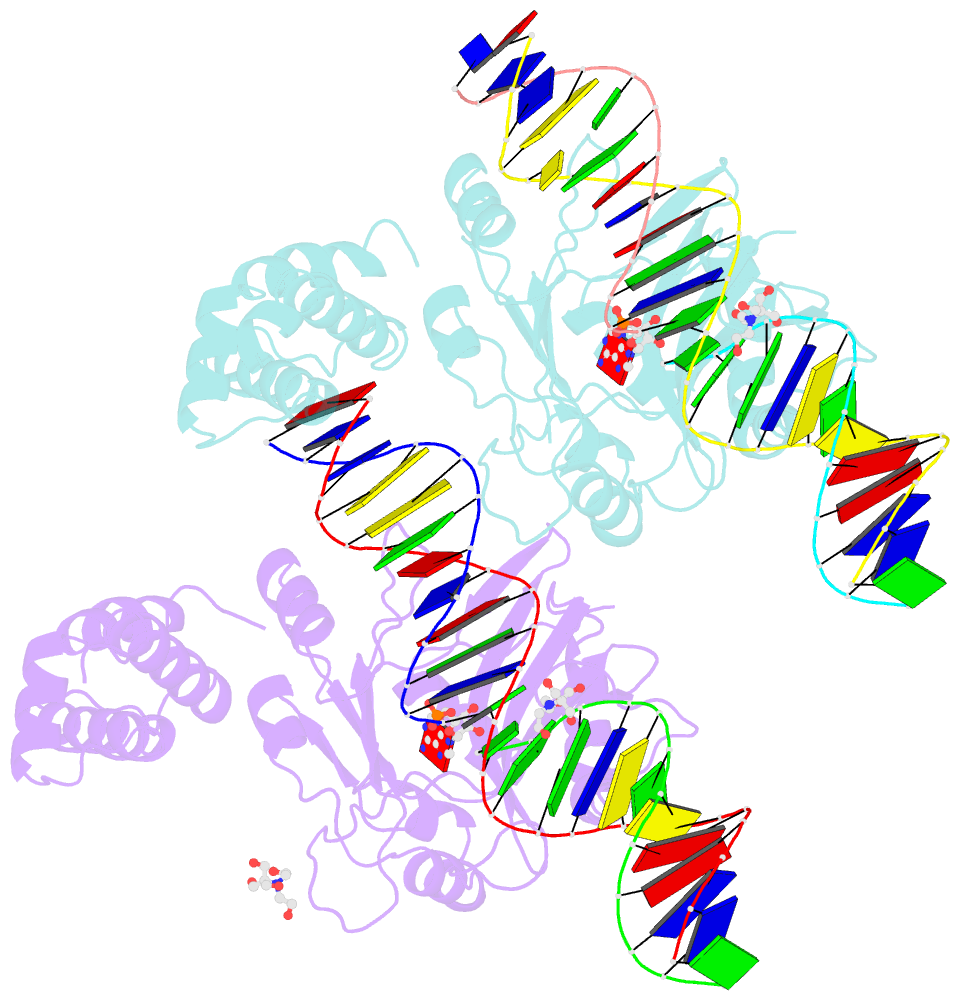
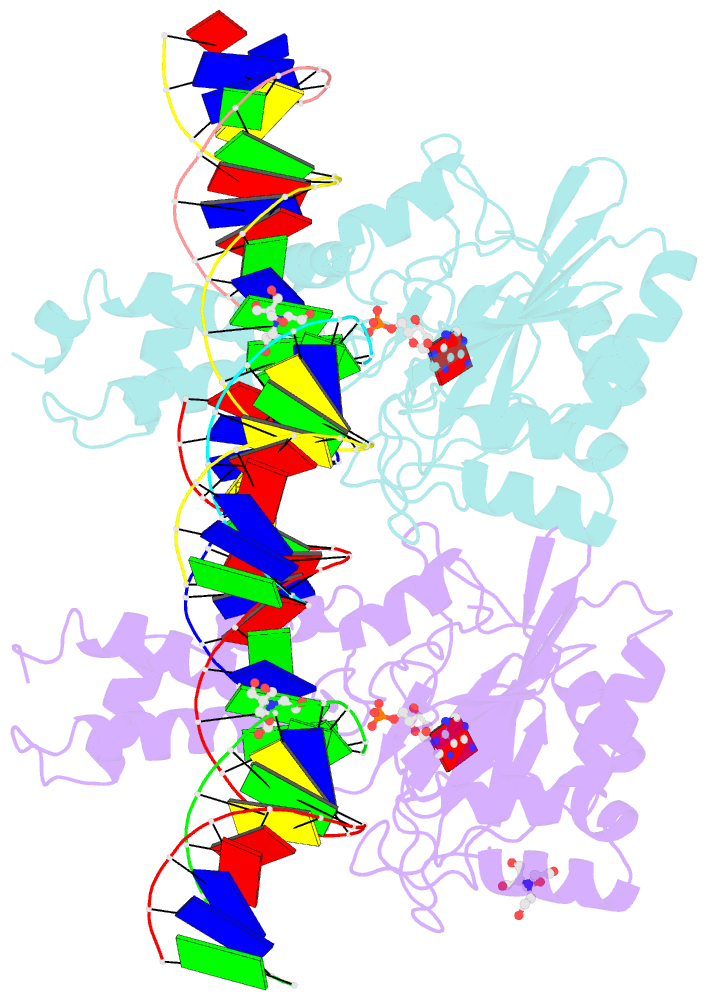
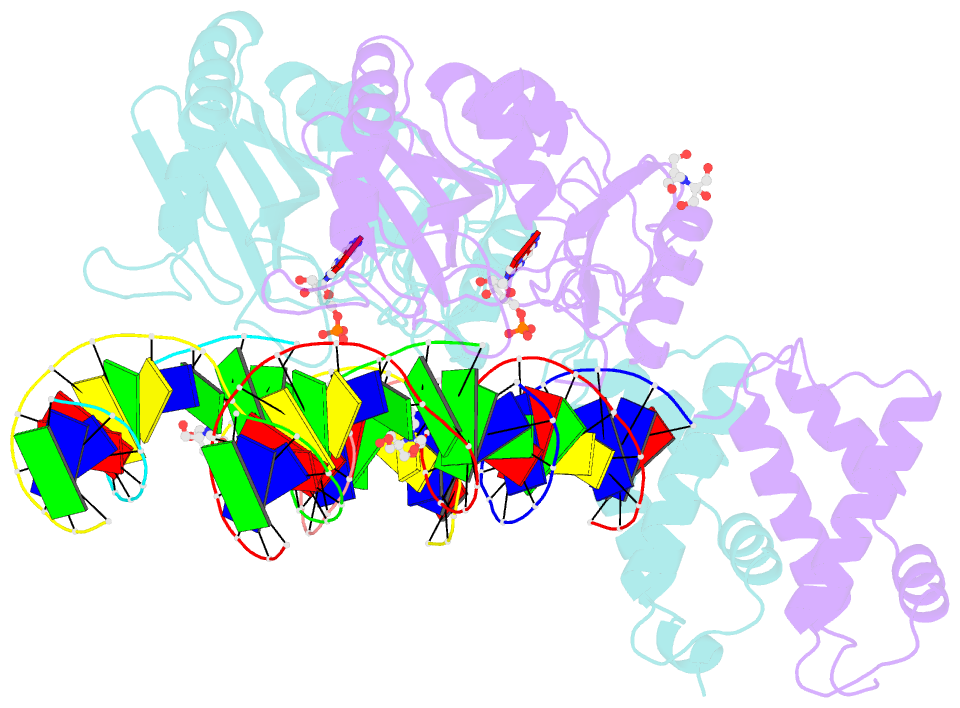
- Structure of t4 RNA ligase 2 with nicked 5'-adenylated nucleic acid duplex containing a 2'-deoxyribonucleotide at the nick
- Reference
- Nandakumar J, Shuman S, Lima CD (2006): "RNA Ligase Structures Reveal the Basis for RNA Specificity and Conformational Changes that Drive Ligation Forward." Cell(Cambridge,Mass.), 127, 71-84. doi: 10.1016/j.cell.2006.08.038.
- Abstract
- T4 RNA ligase 2 (Rnl2) and kinetoplastid RNA editing ligases exemplify a family of RNA repair enzymes that seal 3'OH/5'PO(4) nicks in duplex RNAs via ligase adenylylation (step 1), AMP transfer to the nick 5'PO(4) (step 2), and attack by the nick 3'OH on the 5'-adenylylated strand to form a phosphodiester (step 3). Crystal structures are reported for Rnl2 at discrete steps along this pathway: the covalent Rnl2-AMP intermediate; Rnl2 bound to an adenylylated nicked duplex, captured immediately following step 2; and Rnl2 at an adenylylated nick in a state poised for step 3. These structures illuminate the stereochemistry of nucleotidyl transfer and reveal how remodeling of active-site contacts and conformational changes propel the ligation reaction forward. Mutational analysis and comparison of nick-bound structures of Rnl2 and human DNA ligase I highlight common and divergent themes of substrate recognition that can explain their specialization for RNA versus DNA repair.