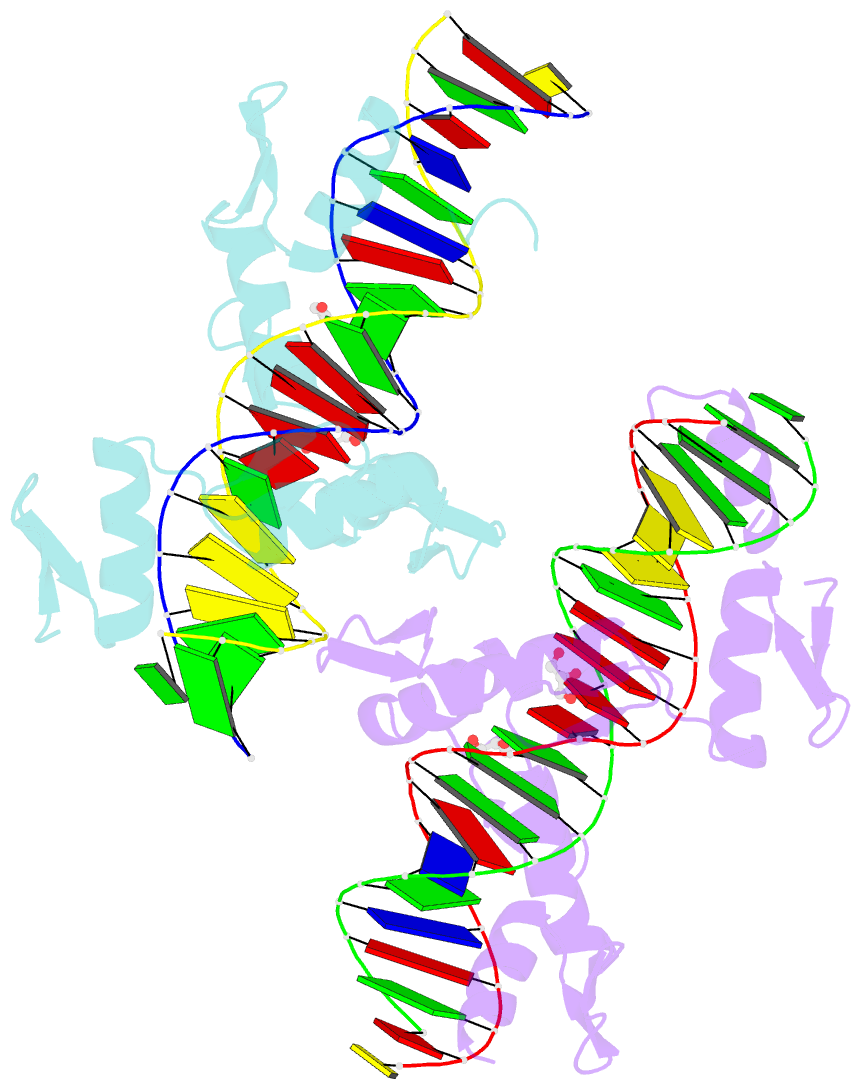
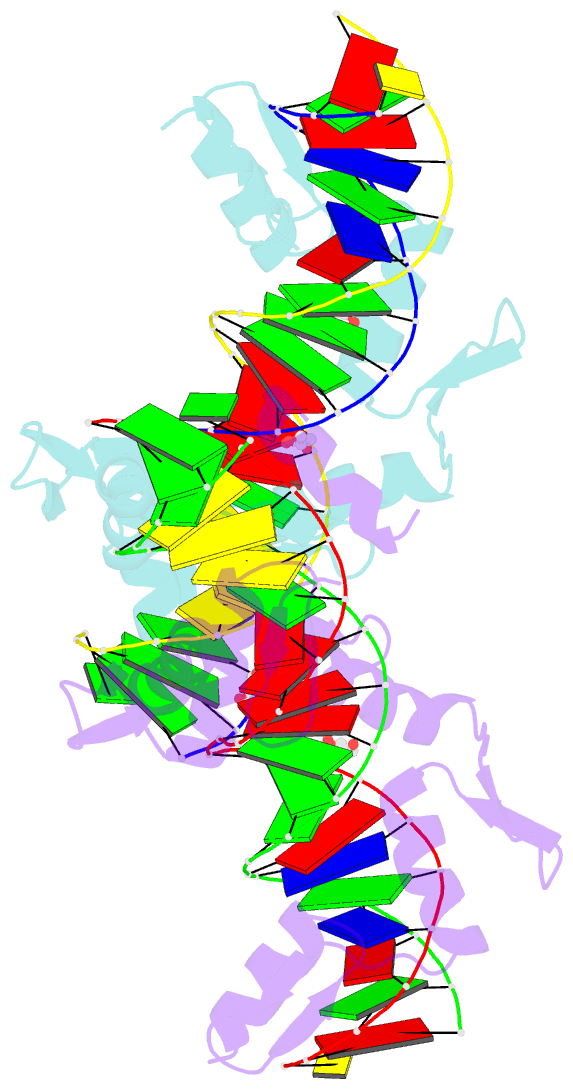
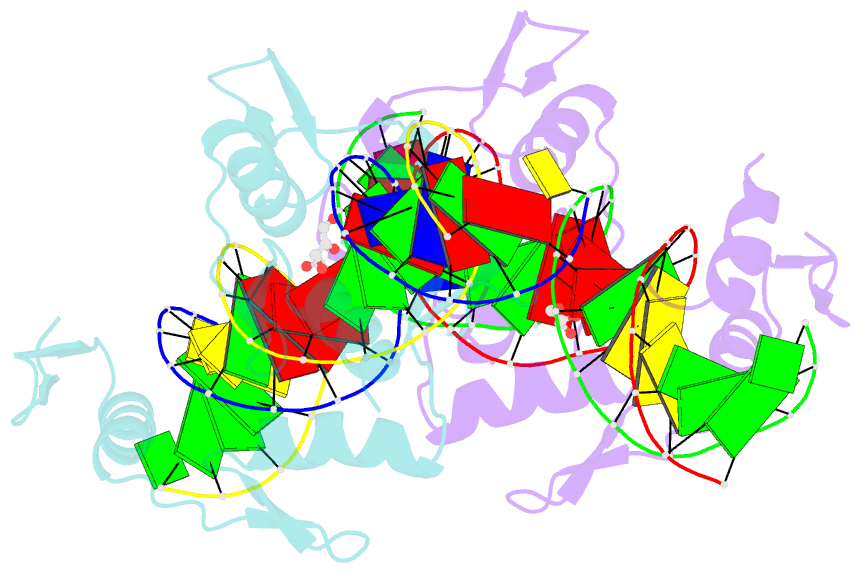
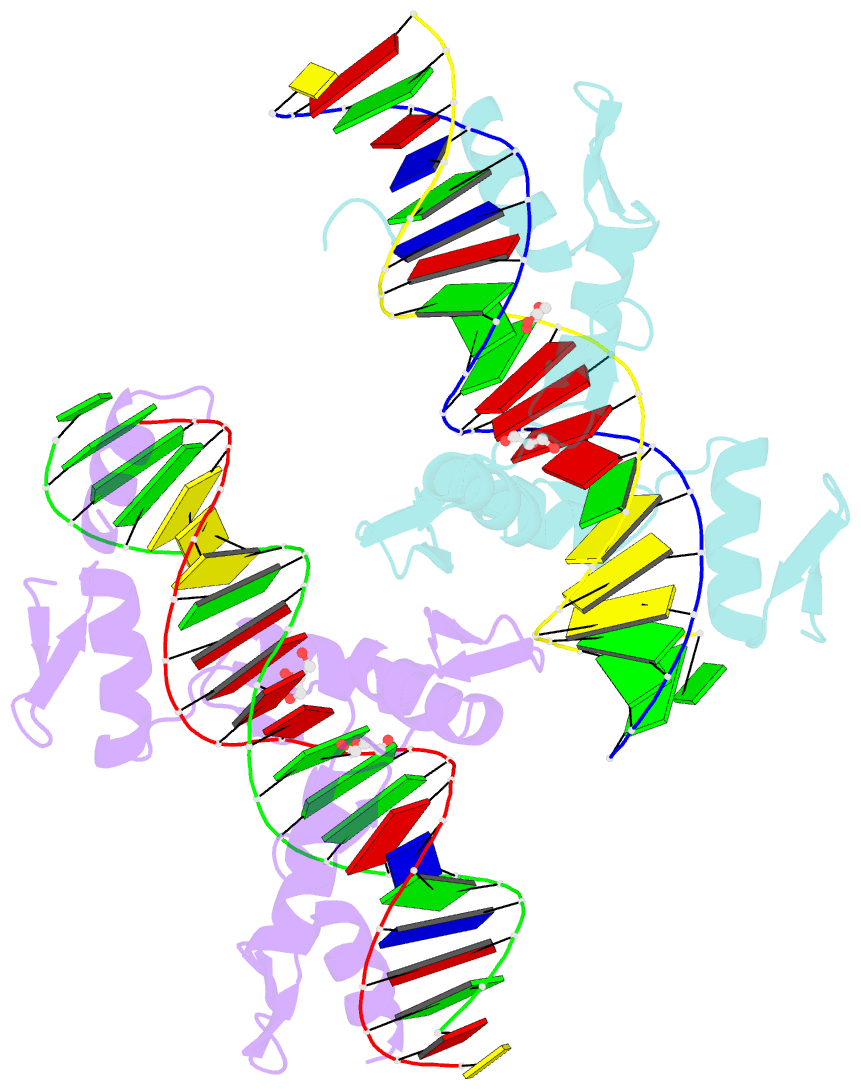
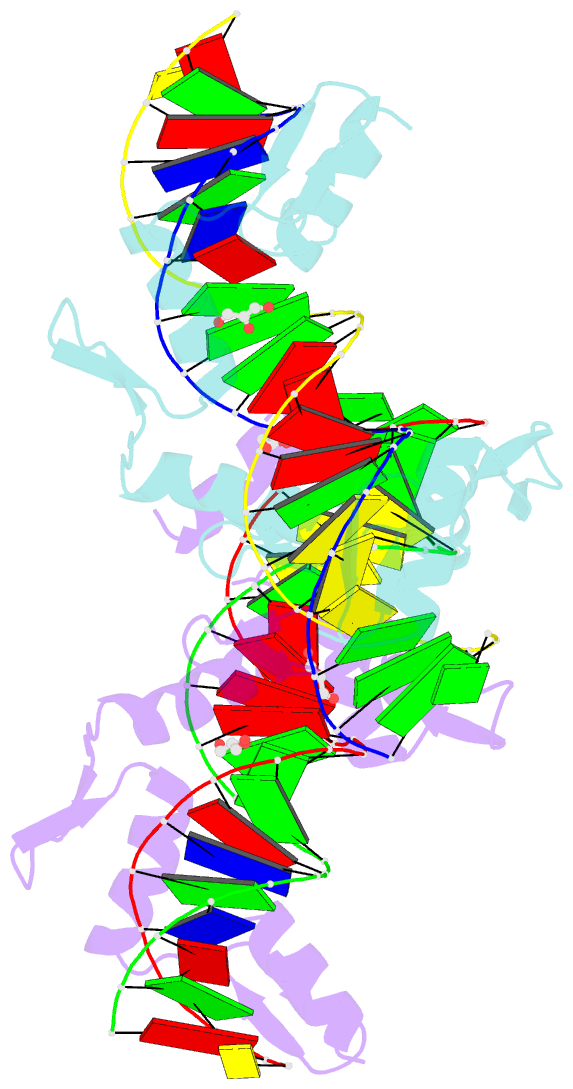
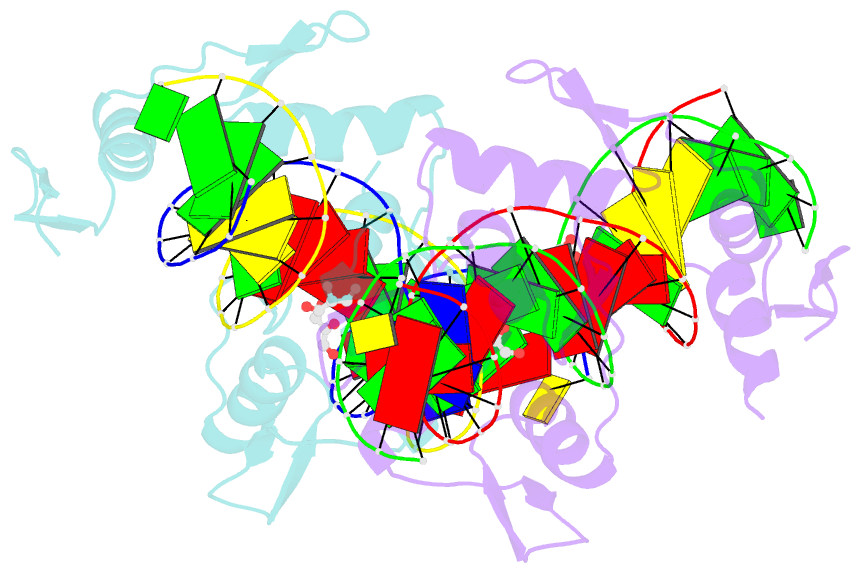
Summary information and primary citation
- PDB-id
- 2i13; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (1.96 Å)
- Summary
- Aart, a six finger zinc finger designed to recognize ann triplets
- Reference
- Segal DJ, Crotty JW, Bhakta MS, Barbas CF, Horton NC (2006): "Structure of Aart, a Designed Six-finger Zinc Finger Peptide, Bound to DNA." J.Mol.Biol., 363, 405-421. doi: 10.1016/j.jmb.2006.08.016.
- Abstract
- Cys2-His2 zinc fingers are one of the most common types of DNA-binding domains. Modifications to zinc-finger binding specificity have recently enabled custom DNA-binding proteins to be designed to a wide array of target sequences. We present here a 1.96 A structure of Aart, a designed six-zinc finger protein, bound to a consensus DNA target site. This is the first structure of a designed protein with six fingers, and was intended to provide insights into the unusual affinity and specificity characteristics of this protein. Most protein-DNA contacts were found to be consistent with expectations, while others were unanticipated or insufficient to explain specificity. Several were unexpectedly mediated by glycerol, water molecules or amino acid-base stacking interactions. These results challenge some conventional concepts of recognition, particularly the finding that triplets containing 5'A, C, or T are typically not specified by direct interaction with the amino acid in position 6 of the recognition helix.