Summary information and primary citation
- PDB-id
- 2i9k; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.65 Å)
- Summary
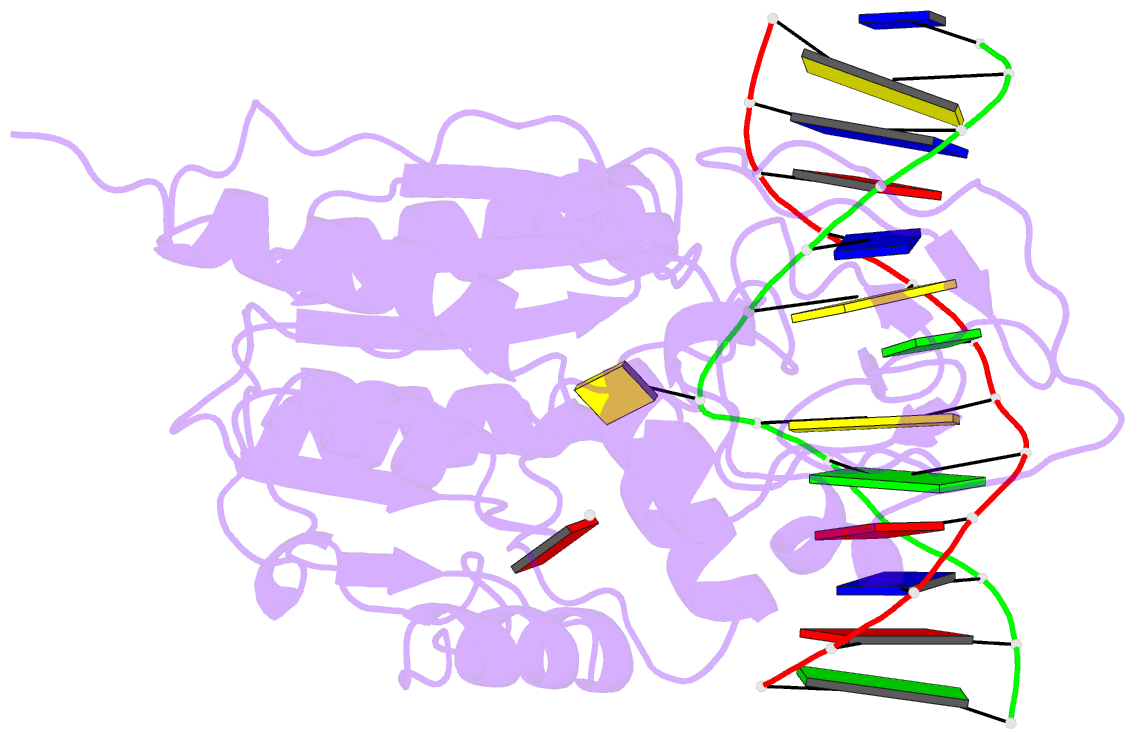
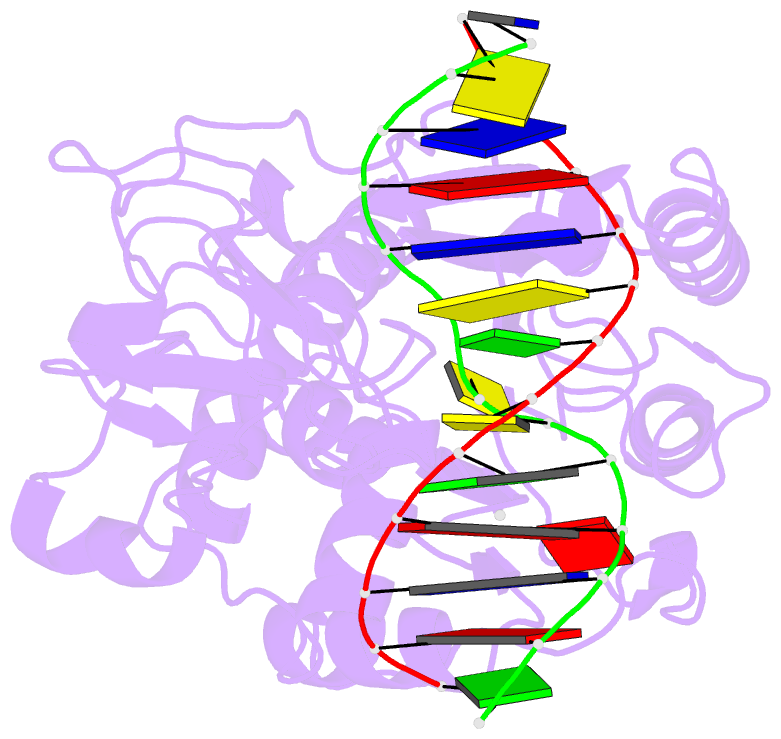
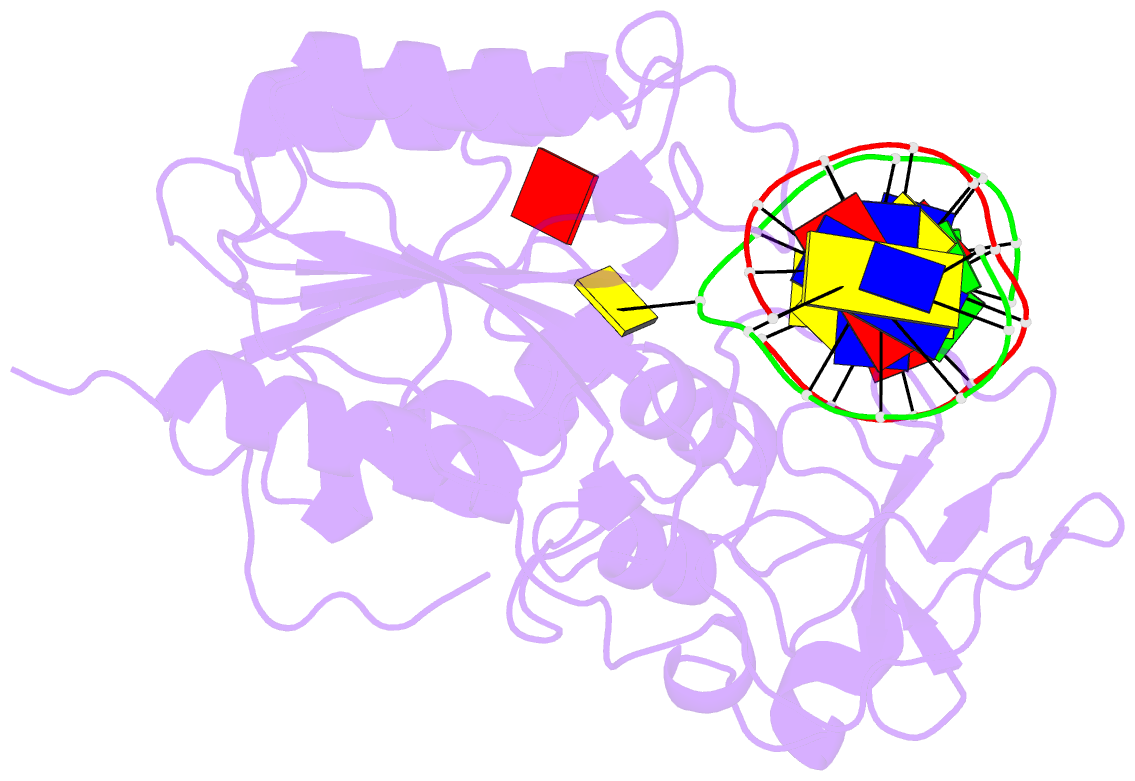
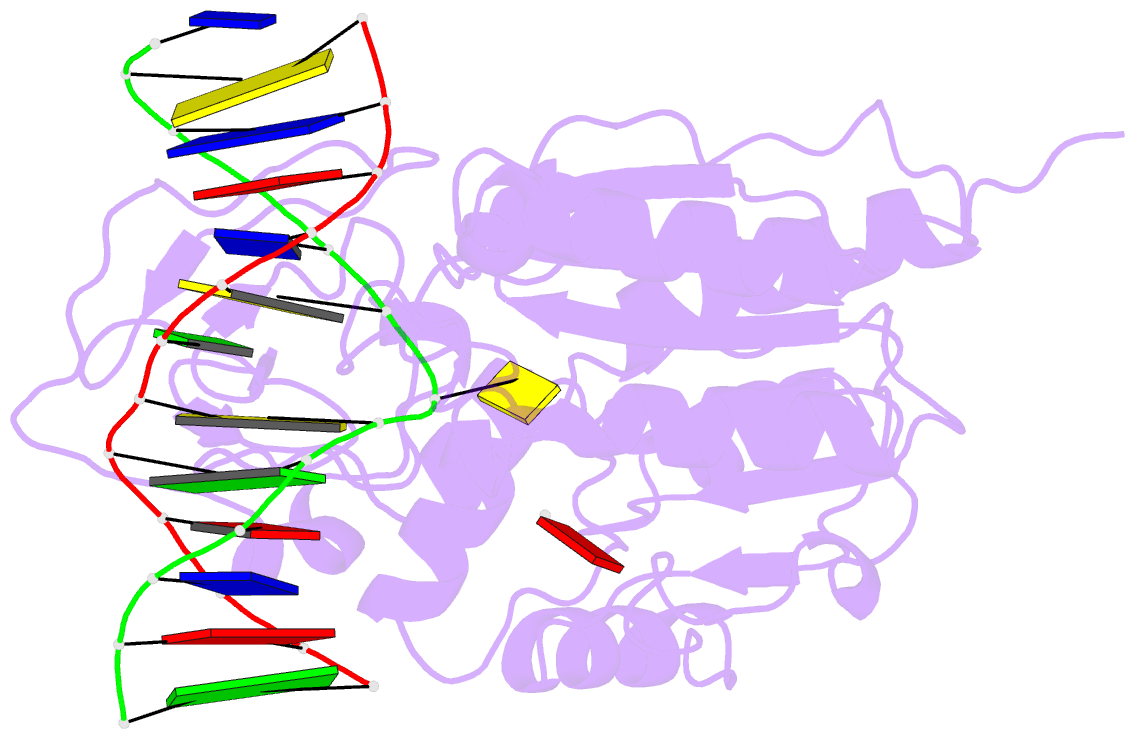
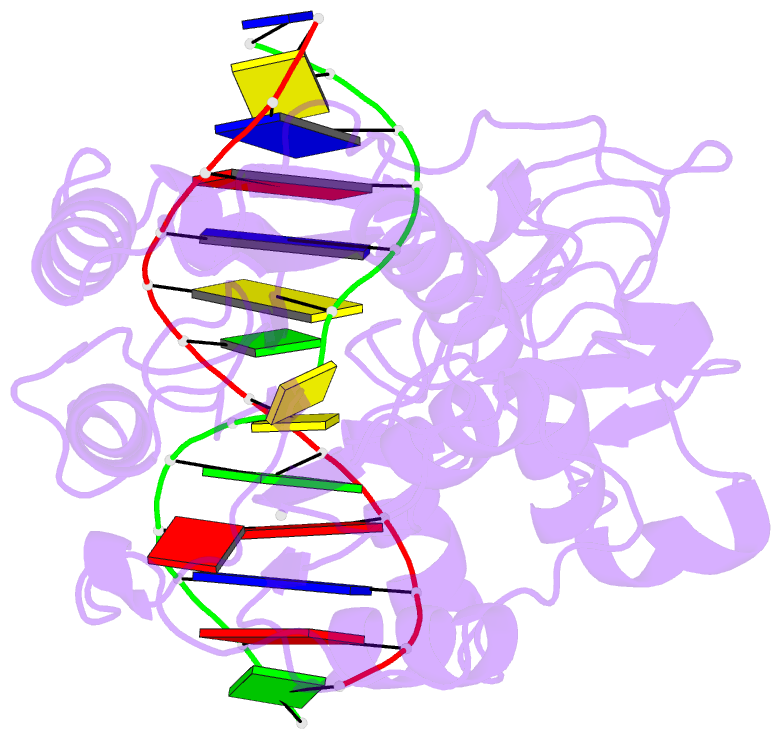
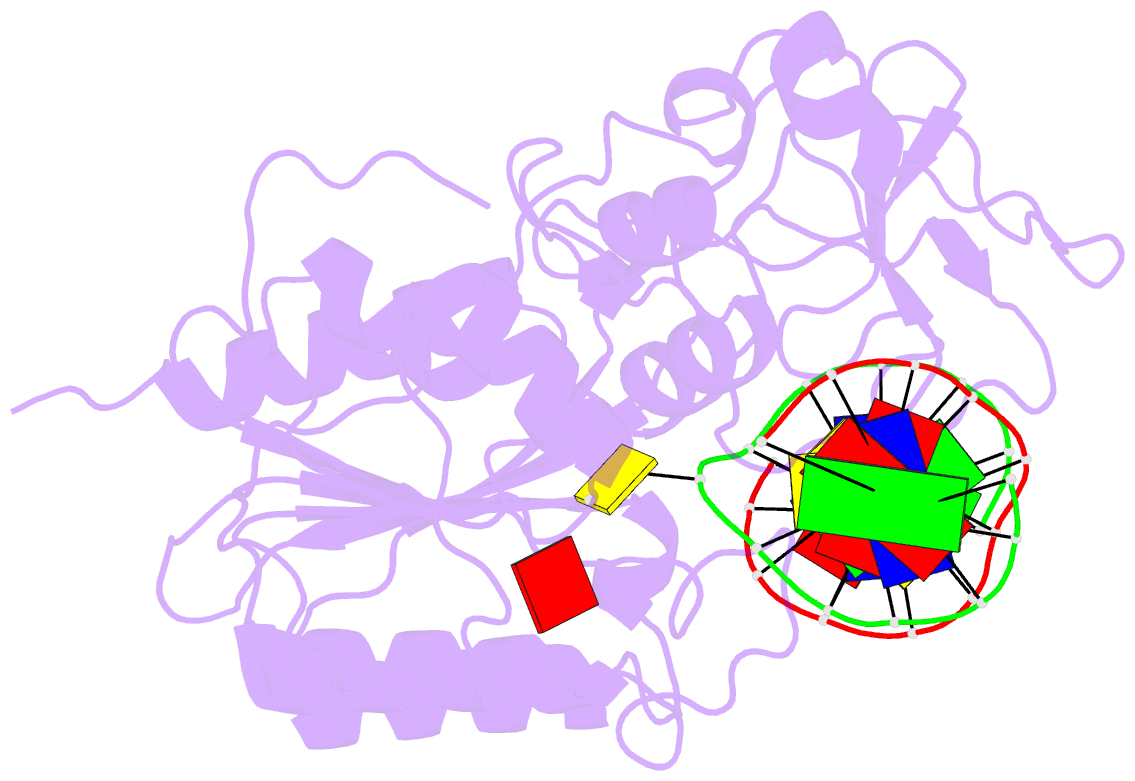
- Engineered extrahelical base destabilization enhances sequence discrimination of DNA methyltransferase m.hhai
- Reference
- Youngblood B, Shieh FK, De Los Rios S, Perona JJ, Reich NO (2006): "Engineered Extrahelical Base Destabilization Enhances Sequence Discrimination of DNA Methyltransferase M.HhaI." J.Mol.Biol., 362, 334-346. doi: 10.1016/j.jmb.2006.07.031.
- Abstract
- Improved sequence specificity of the DNA cytosine methyltransferase HhaI was achieved by disrupting interactions at a hydrophobic interface between the active site of the enzyme and a highly conserved flexible loop. Transient fluorescence experiments show that mutations disrupting this interface destabilize the positioning of the extrahelical, "flipped" cytosine base within the active site. The ternary crystal structure of the F124A M.HhaI bound to cognate DNA and the cofactor analogue S-adenosyl-l-homocysteine shows an increase in cavity volume between the flexible loop and the core of the enzyme. This cavity disrupts the interface between the loop and the active site, thereby destabilizing the extrahelical target base. The favored partitioning of the base-flipped enzyme-DNA complex back to the base-stacked intermediate results in the mutant enzyme discriminating better than the wild-type enzyme against non-cognate sites. Building upon the concepts of kinetic proofreading and our understanding of M.HhaI, we describe how a 16-fold specificity enhancement achieved with a double mutation at the loop/active site interface is acquired through destabilization of intermediates prior to methyltransfer rather than disruption of direct interactions between the enzyme and the substrate for M.HhaI.