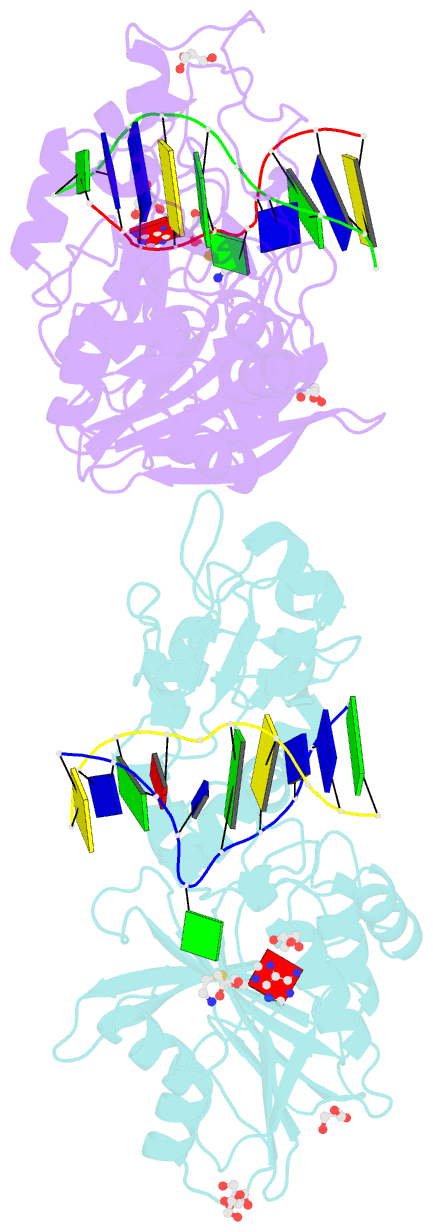Summary information and primary citation
- PDB-id
- 2ibt; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (1.7 Å)
- Summary
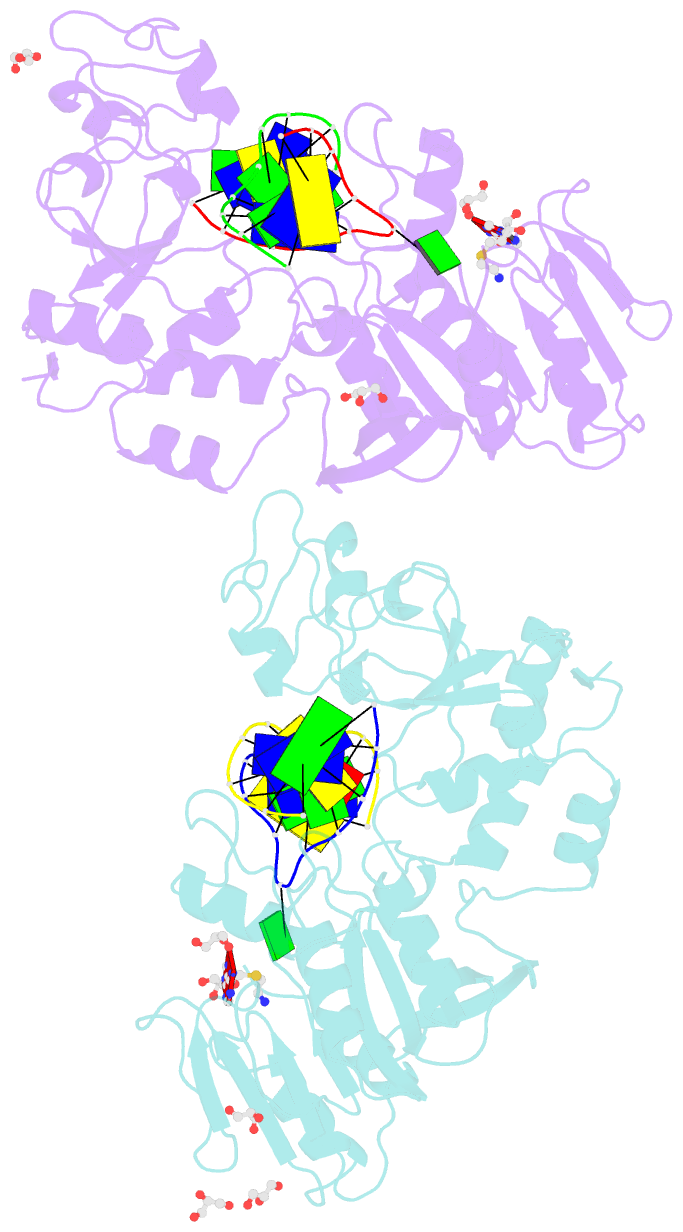
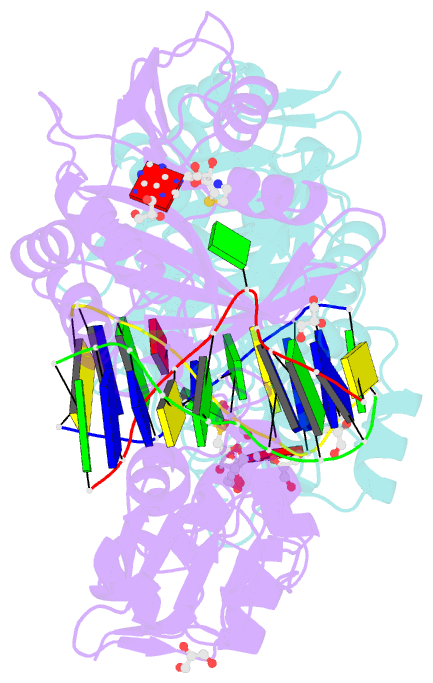
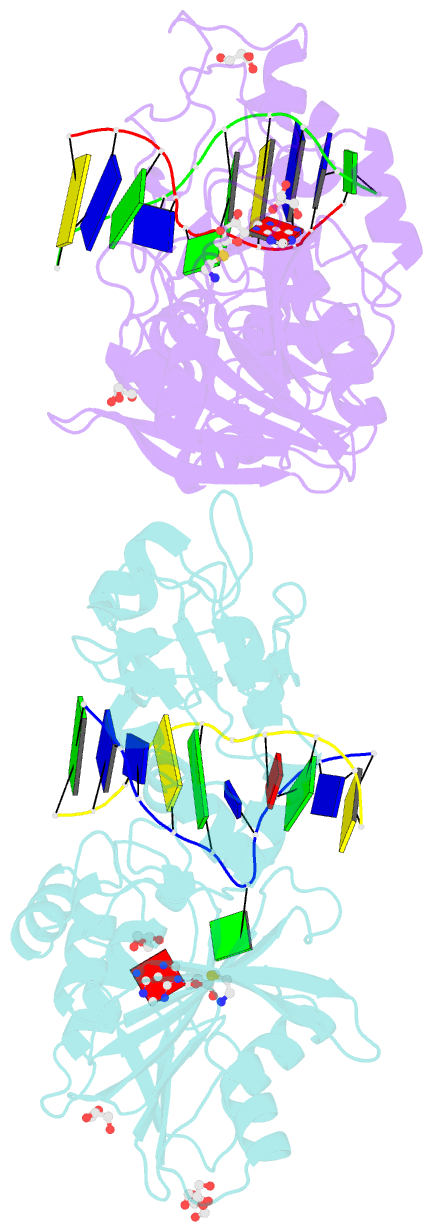
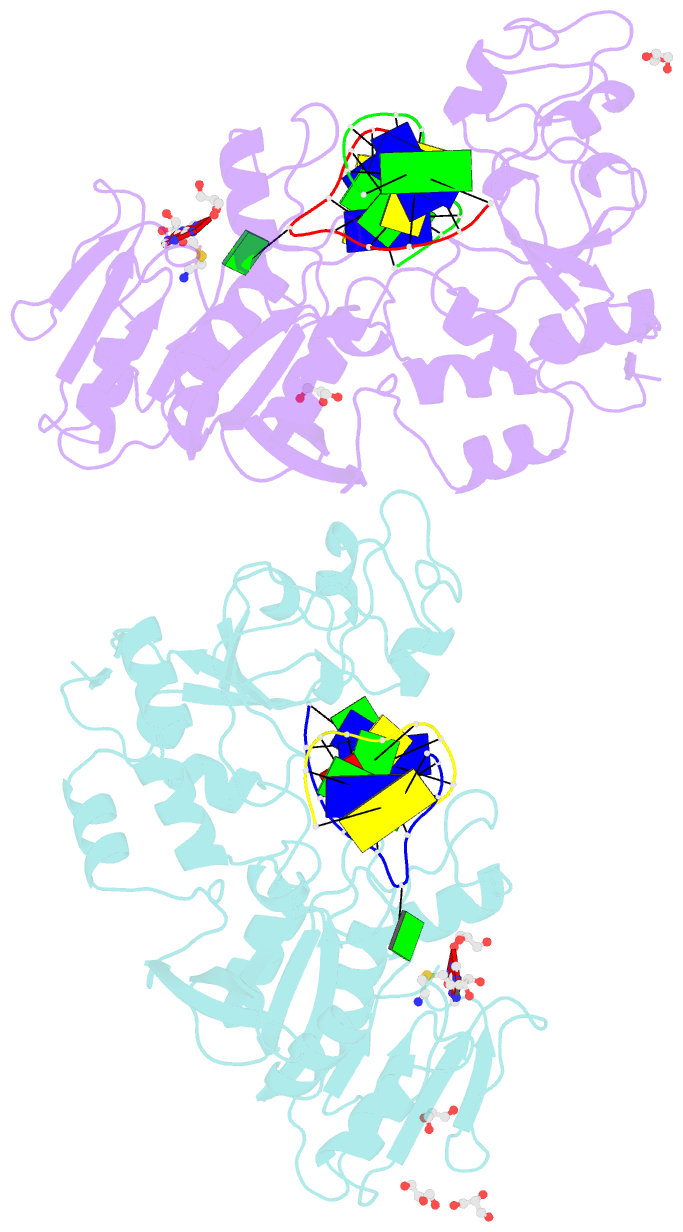
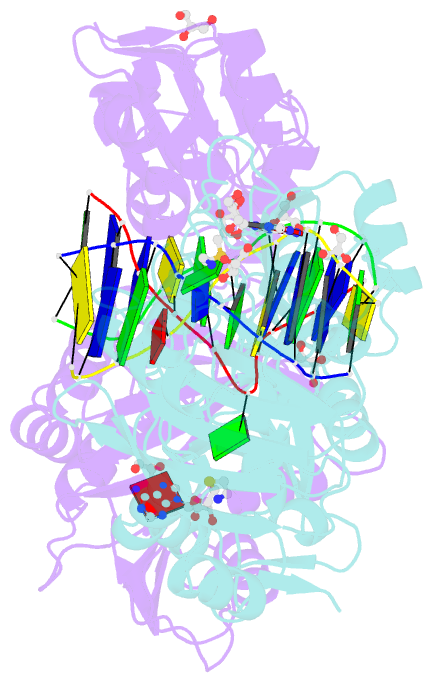
- Crystal structure of the adenine-specific DNA methyltransferase m.taqi complexed with the cofactor analog aeta and a 10 bp DNA containing 2-aminopurine at the target position and an abasic site analog at the target base partner position
- Reference
- Lenz T, Bonnist EYM, Pljevaljcic G, Neely RK, Dryden DTF, Scheidig AJ, Jones AC, Weinhold E (2007): "2-Aminopurine Flipped into the Active Site of the Adenine-Specific DNA Methyltransferase M.TaqI: Crystal Structures and Time-Resolved Fluorescence." J.Am.Chem.Soc., 129, 6240-6248. doi: 10.1021/ja069366n.
- Abstract
- We report the crystal structure of the DNA adenine-N6 methyltransferase, M.TaqI, complexed with DNA, showing the fluorescent adenine analog, 2-aminopurine, flipped out of the DNA helix and occupying virtually the same position in the active site as the natural target adenine. Time-resolved fluorescence spectroscopy of the crystalline complex faithfully reports this state: base flipping is accompanied by the loss of the very short ( approximately 50 ps) lifetime component associated with fully base-stacked 2-aminopurine in DNA, and 2-aminopurine is subject to considerable quenching by pi-stacking interactions with Tyr108 in the catalytic motif IV (NPPY). This proves 2-aminopurine to be an excellent probe for studying base flipping by M.TaqI and suggests similar quenching in the active sites of DNA and RNA adenine-N6 as well as DNA cytosine-N4 methyltransferases sharing the conserved motif IV. In solution, the same distinctive fluorescence response confirms complete destacking from DNA and is also observed when the proposed key residue for base flipping by M.TaqI, the target base partner thymine, is substituted by an abasic site analog. The corresponding cocrystal structure shows 2-aminopurine in the active site of M.TaqI, demonstrating that the partner thymine is not essential for base flipping. However, in this structure, a shift of the 3' neighbor of the target base into the vacancy left after base flipping is observed, apparently replicating a stabilizing role of the missing partner thymine. Time-resolved fluorescence and acrylamide quenching measurements of M.TaqI complexes in solution provide evidence for an alternative binding site for the extra-helical target base within M.TaqI and suggest that the partner thymine assists in delivering the target base into the active site.