Summary information and primary citation
- PDB-id
- 2ihn; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.0 Å)
- Summary
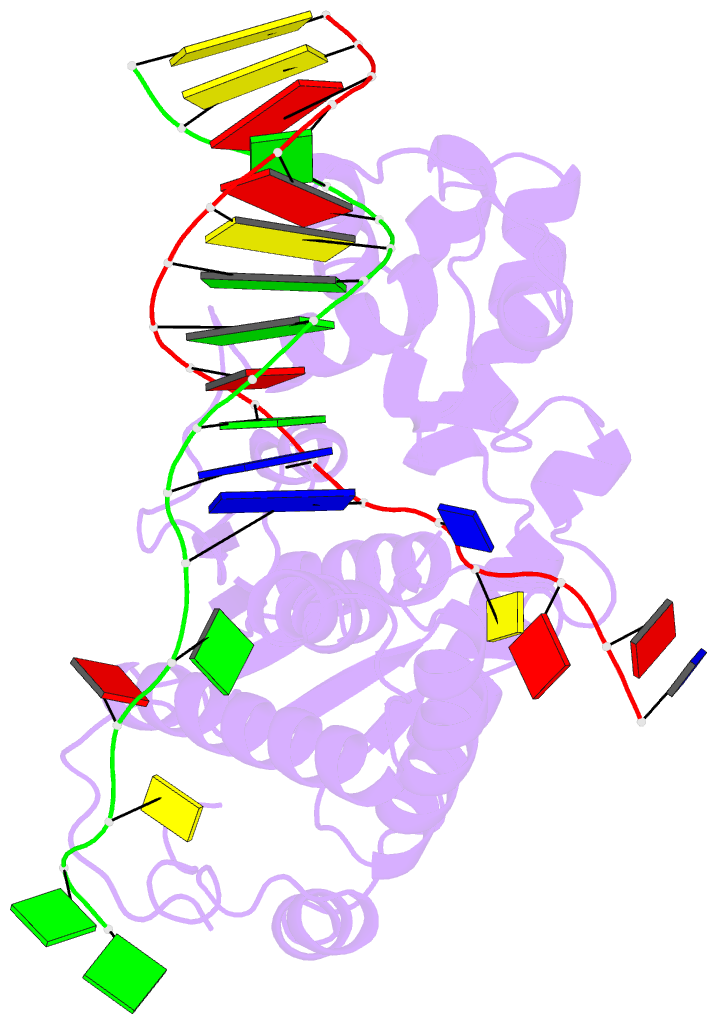
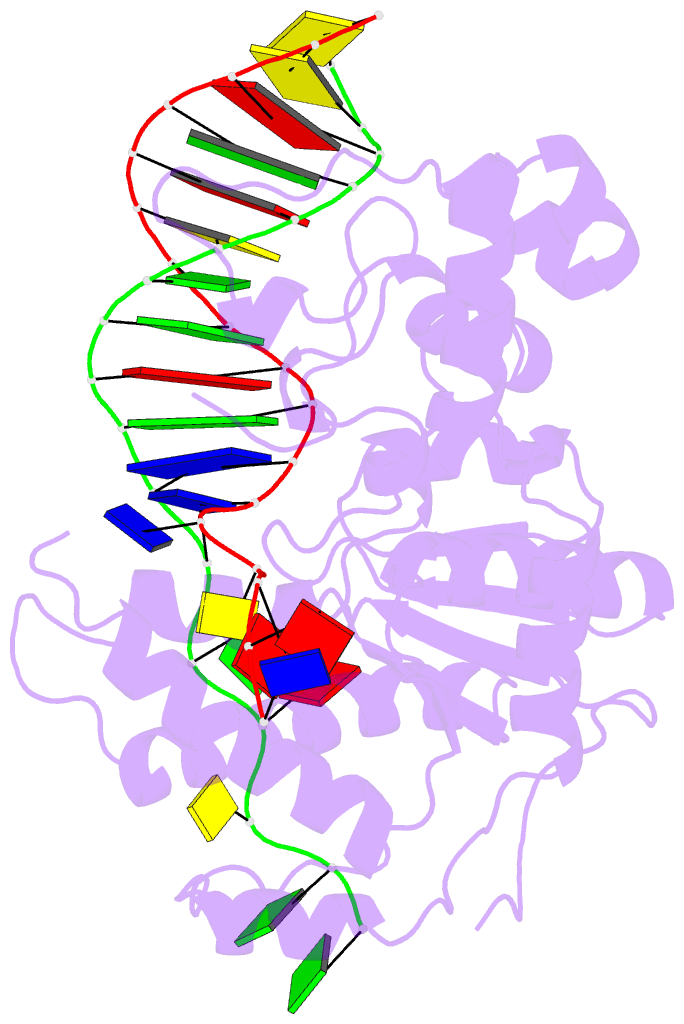
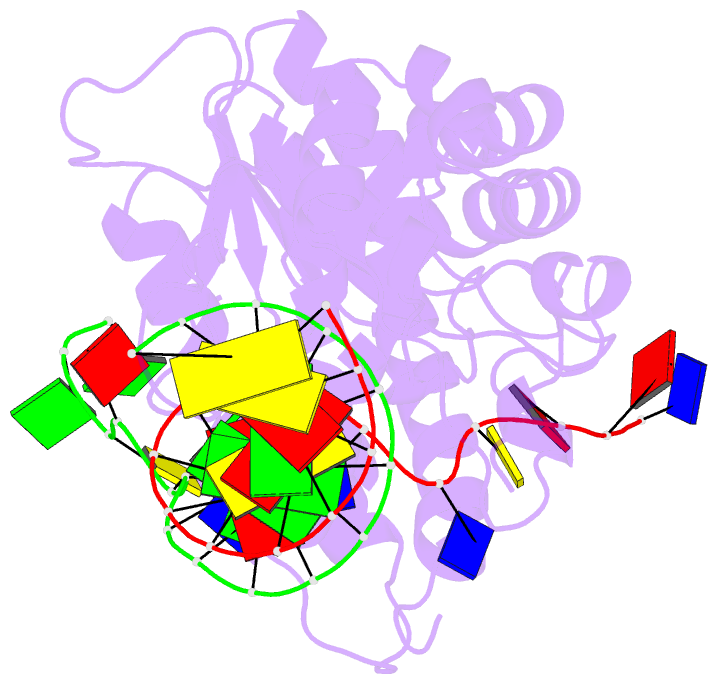
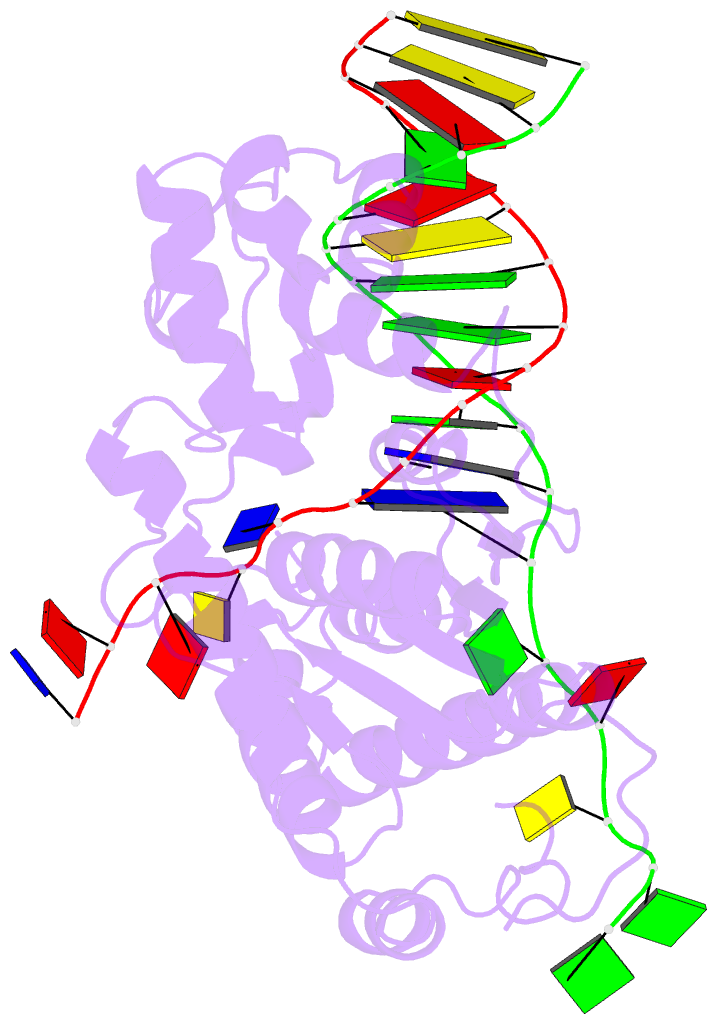
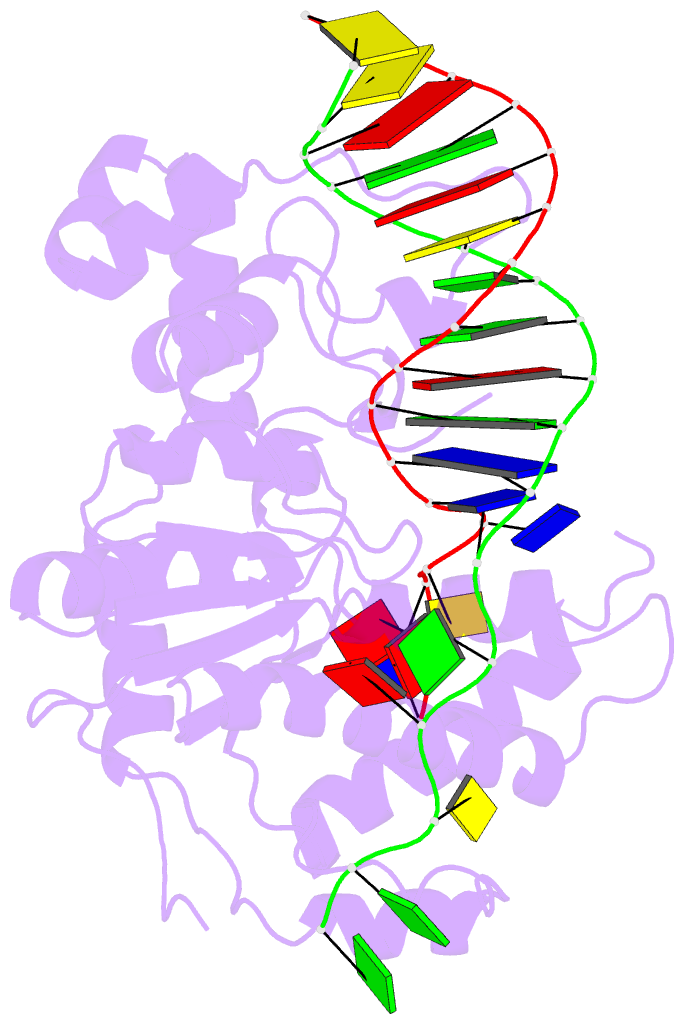
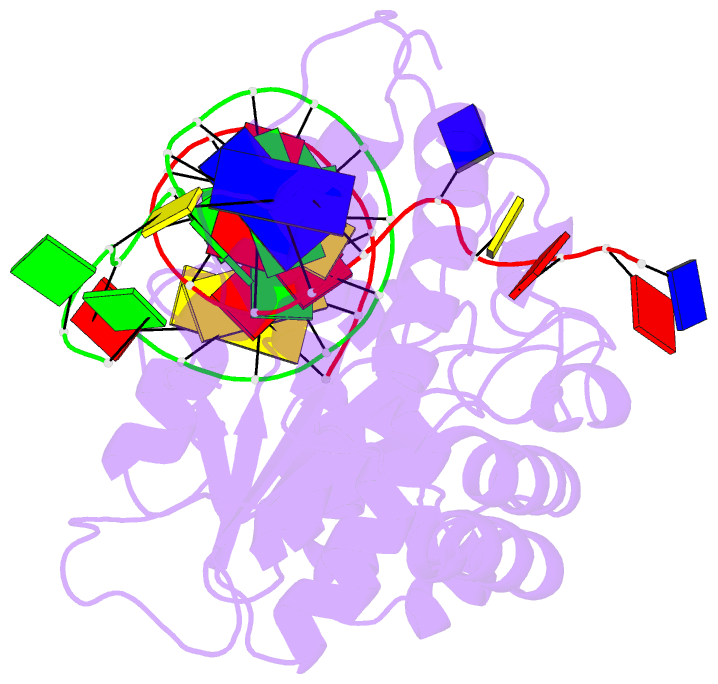
- Co-crystal of bacteriophage t4 rnase h with a fork DNA substrate
- Reference
- Devos JM, Tomanicek SJ, Jones CE, Nossal NG, Mueser TC (2007): "Crystal structure of bacteriophage T4 5' nuclease in complex with a branched DNA reveals how FEN-1 family nucleases bind their substrates." J.Biol.Chem., 282, 31713-31724. doi: 10.1074/jbc.M703209200.
- Abstract
- Bacteriophage T4 RNase H, a flap endonuclease-1 family nuclease, removes RNA primers from lagging strand fragments. It has both 5' nuclease and flap endonuclease activities. Our previous structure of native T4 RNase H (PDB code 1TFR) revealed an active site composed of highly conserved Asp residues and two bound hydrated magnesium ions. Here, we report the crystal structure of T4 RNase H in complex with a fork DNA substrate bound in its active site. This is the first structure of a flap endonuclease-1 family protein with its complete branched substrate. The fork duplex interacts with an extended loop of the helix-hairpin-helix motif class 2. The 5' arm crosses over the active site, extending below the bridge (helical arch) region. Cleavage assays of this DNA substrate identify a primary cut site 7-bases in from the 5' arm. The scissile phosphate, the first bond in the duplex DNA adjacent to the 5' arm, lies above a magnesium binding site. The less ordered 3' arm reaches toward the C and N termini of the enzyme, which are binding sites for T4 32 protein and T4 45 clamp, respectively. In the crystal structure, the scissile bond is located within the double-stranded DNA, between the first two duplex nucleotides next to the 5' arm, and lies above a magnesium binding site. This complex provides important insight into substrate recognition and specificity of the flap endonuclease-1 enzymes.