Summary information and primary citation
- PDB-id
- 2ja5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase
- Method
- X-ray (3.8 Å)
- Summary
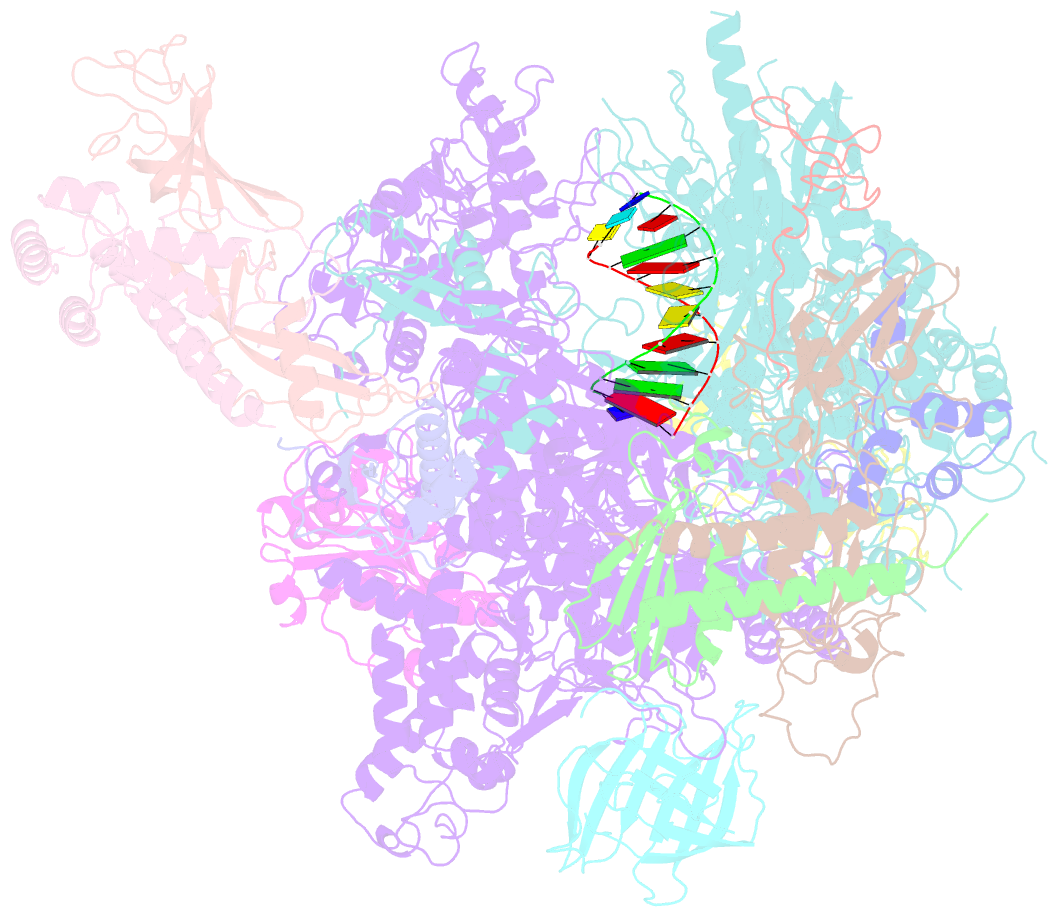
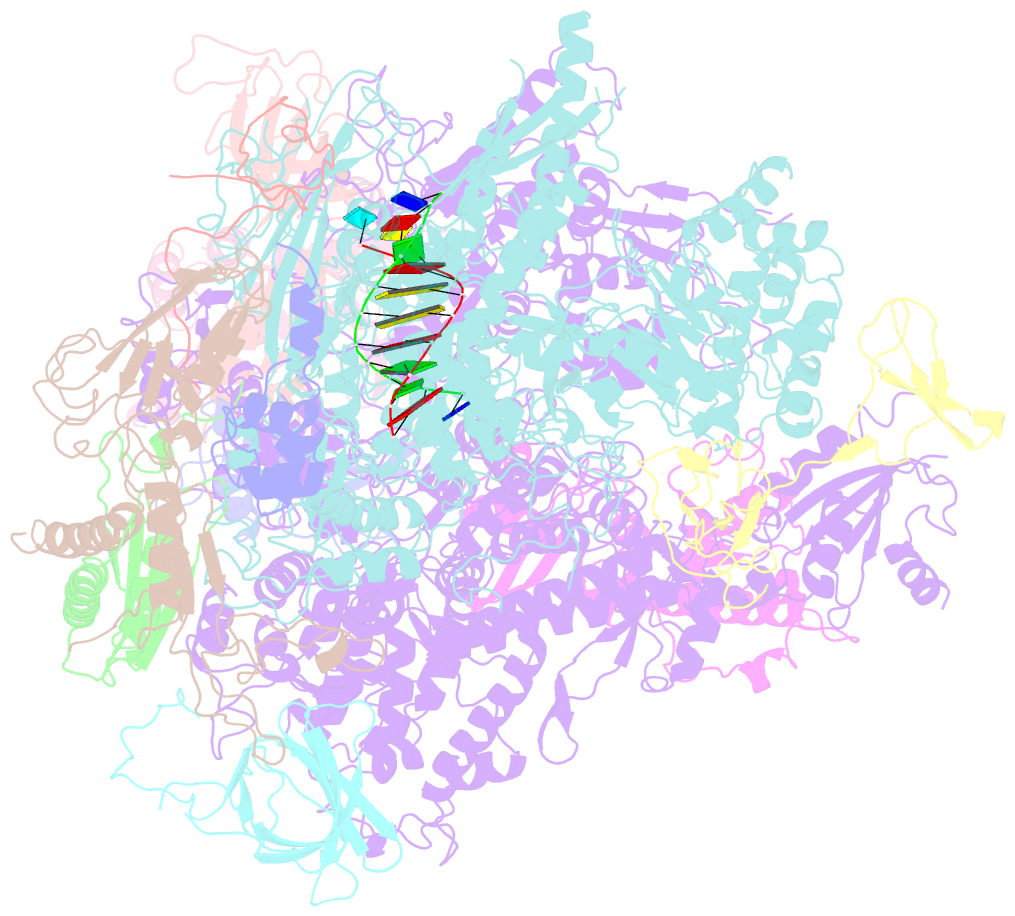
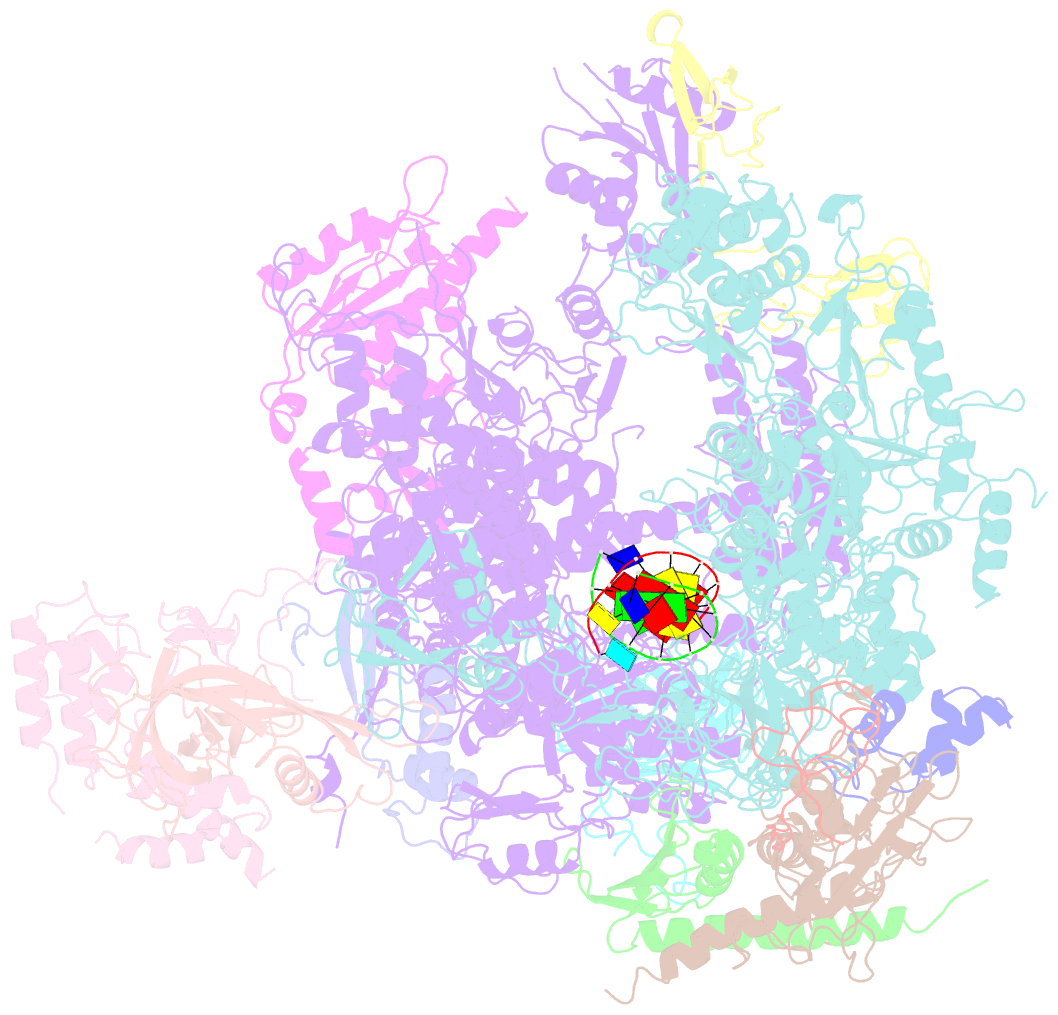
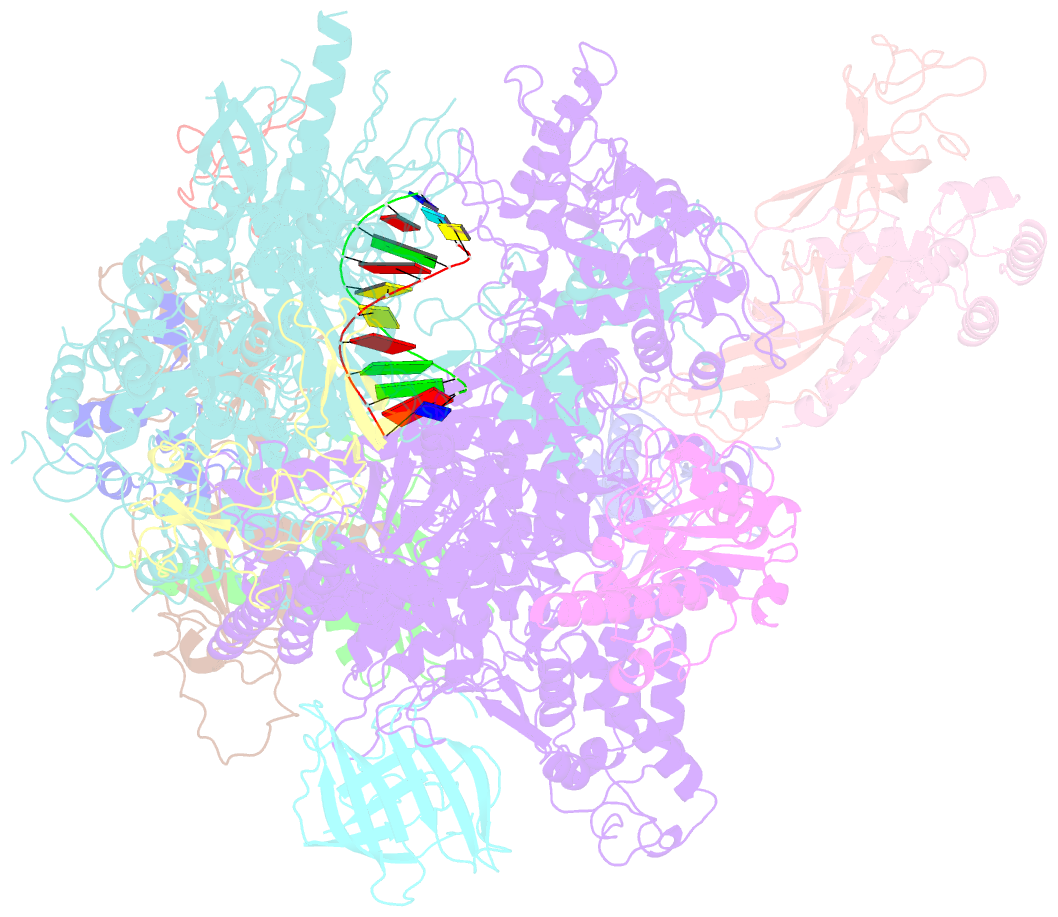
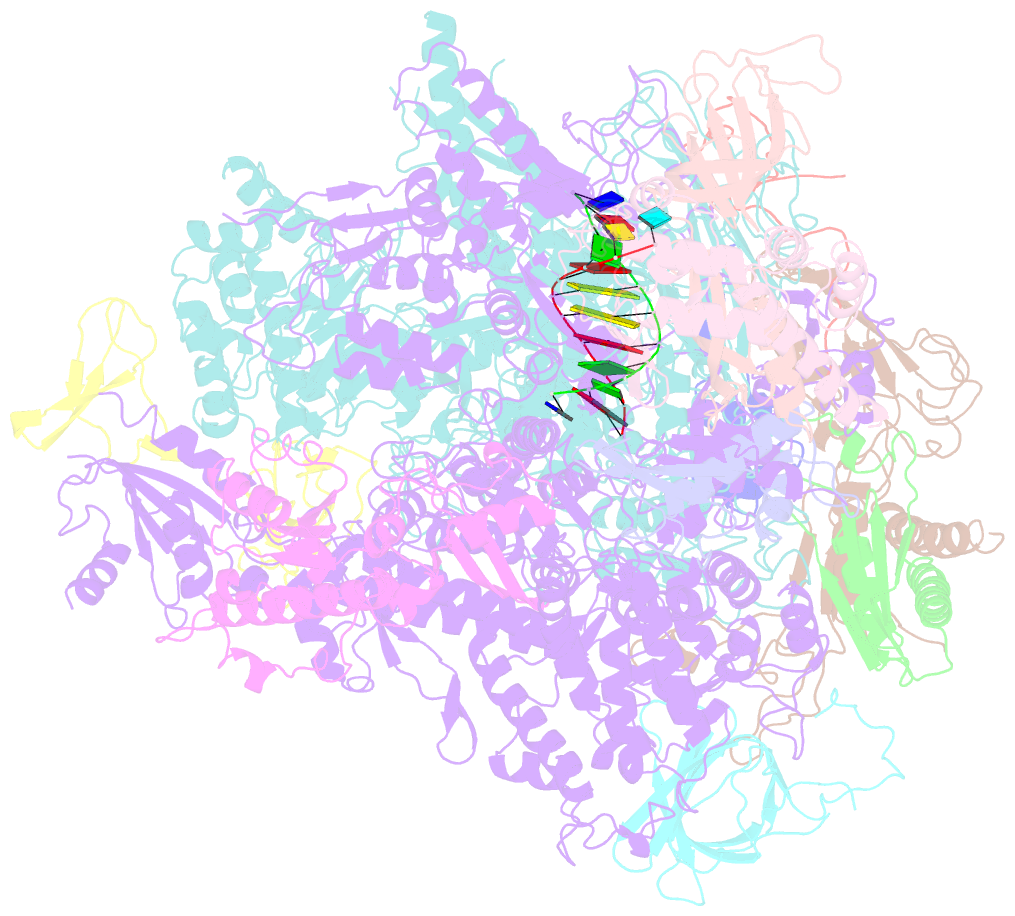
- Cpd lesion containing RNA polymerase ii elongation complex a
- Reference
- Brueckner F, Hennecke U, Carell T, Cramer P (2007): "Cpd Damage Recognition by Transcribing RNA Polymerase II." Science, 315, 859. doi: 10.1126/SCIENCE.1135400.
- Abstract
- Cells use transcription-coupled repair (TCR) to efficiently eliminate DNA lesions such as ultraviolet light-induced cyclobutane pyrimidine dimers (CPDs). Here we present the structure-based mechanism for the first step in eukaryotic TCR, CPD-induced stalling of RNA polymerase (Pol) II. A CPD in the transcribed strand slowly passes a translocation barrier and enters the polymerase active site. The CPD 5'-thymine then directs uridine misincorporation into messenger RNA, which blocks translocation. Artificial replacement of the uridine by adenosine enables CPD bypass; thus, Pol II stalling requires CPD-directed misincorporation. In the stalled complex, the lesion is inaccessible, and the polymerase conformation is unchanged. This is consistent with nonallosteric recruitment of repair factors and excision of a lesion-containing DNA fragment in the presence of Pol II.