Summary information and primary citation
- PDB-id
- 2jea; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.33 Å)
- Summary
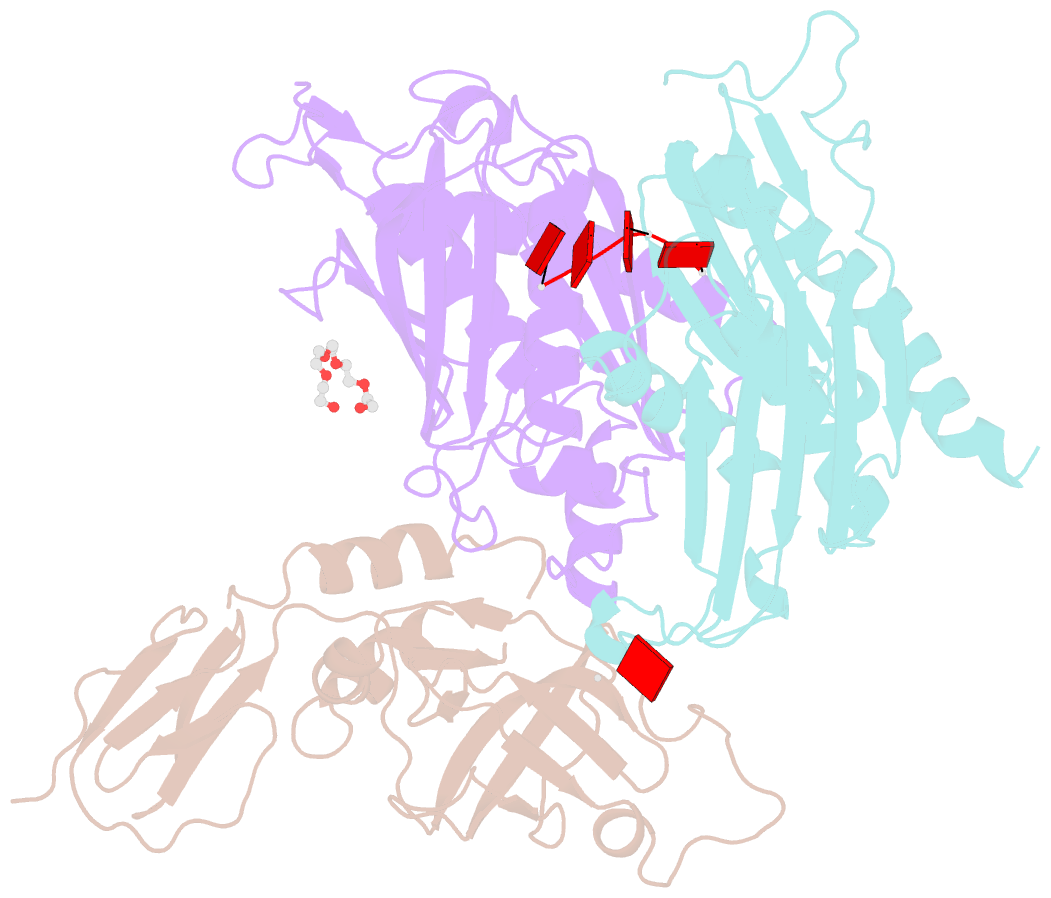
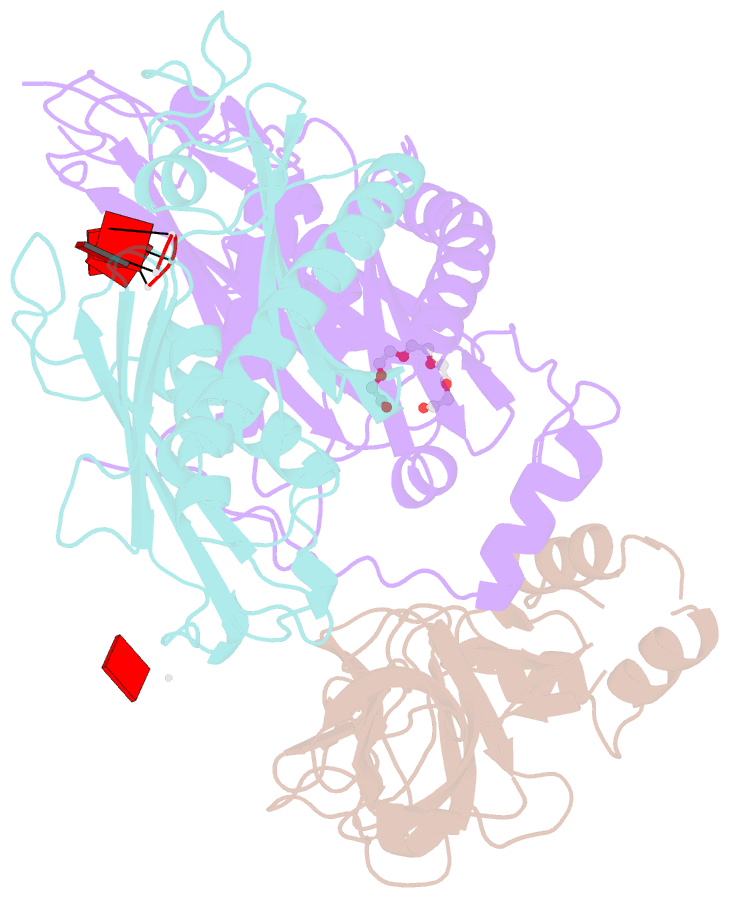
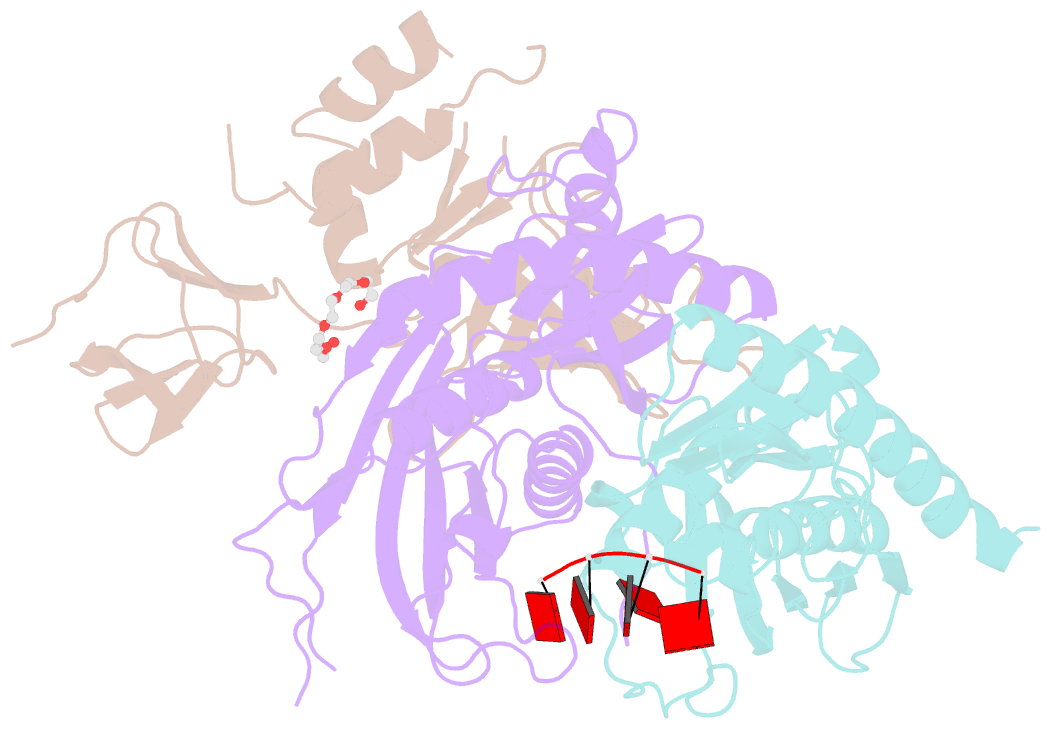
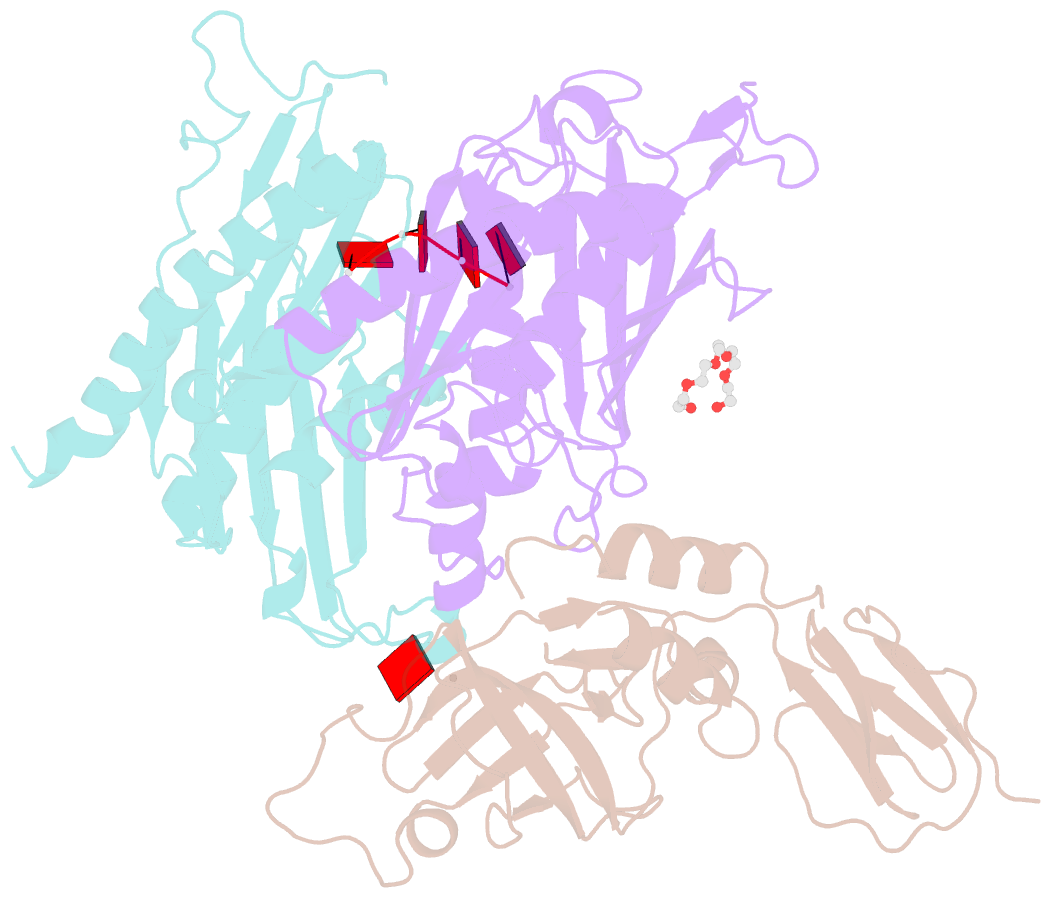
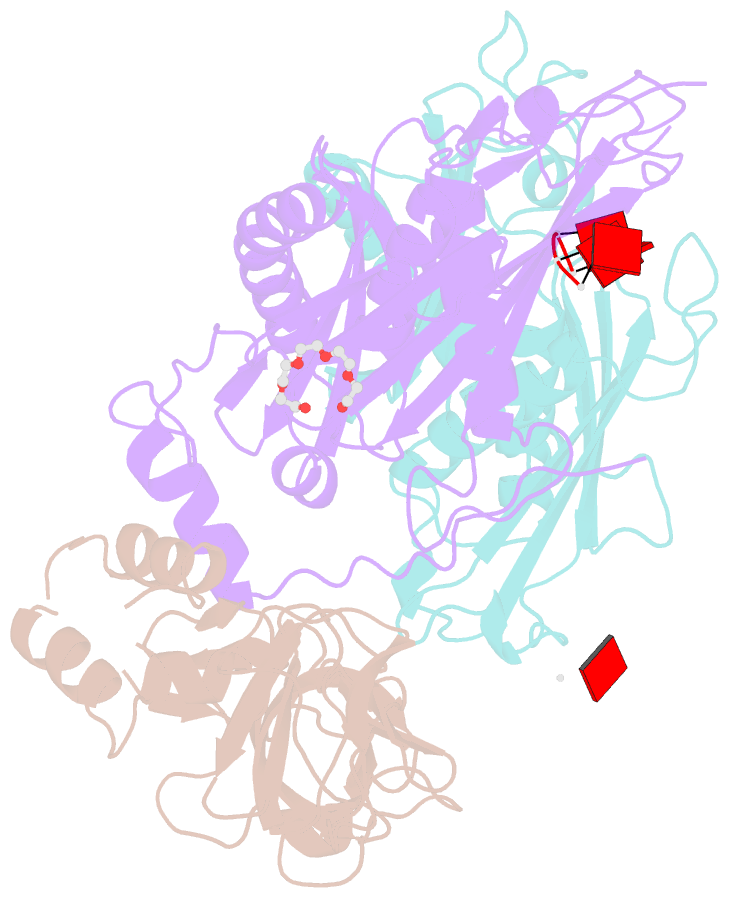
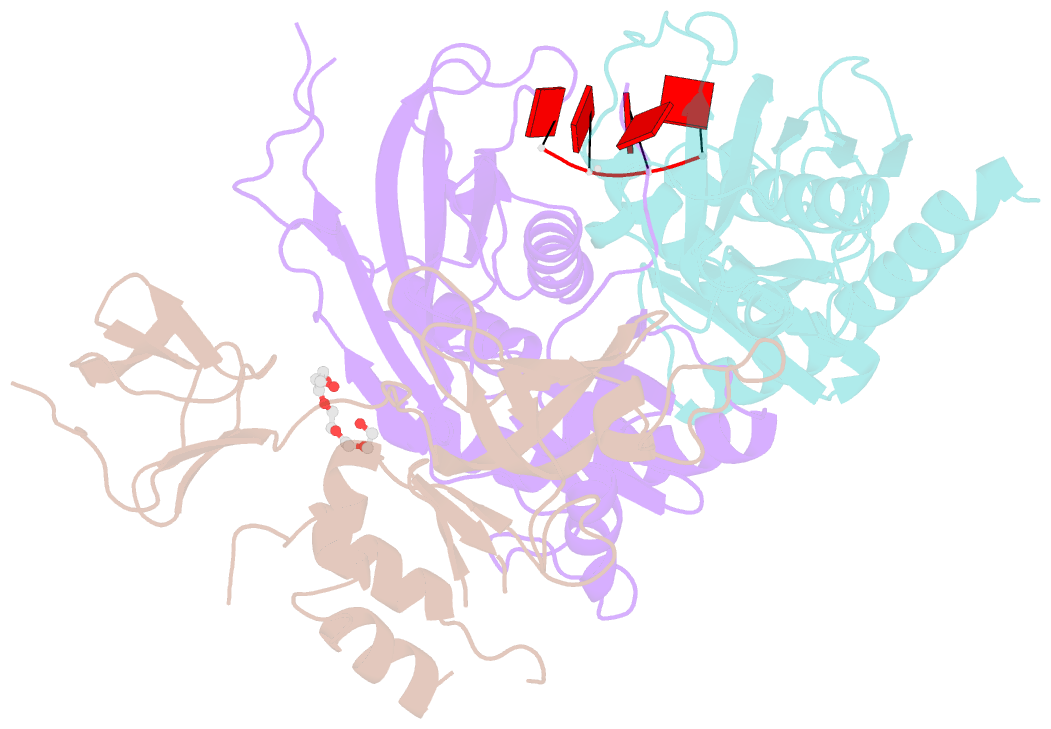
- Structure of a 9-subunit archaeal exosome bound to RNA
- Reference
- Lorentzen E, Dziembowski A, Lindner D, Seraphin B, Conti E (2007): "RNA Channelling by the Archaeal Exosome." Embo Rep., 8, 470. doi: 10.1038/SJ.EMBOR.7400945.
- Abstract
- Exosomes are complexes containing 3' --> 5' exoribonucleases that have important roles in processing, decay and quality control of various RNA molecules. Archaeal exosomes consist of a hexameric core of three active RNase PH subunits (ribosomal RNA processing factor (Rrp)41) and three inactive RNase PH subunits (Rrp42). A trimeric ring of subunits with putative RNA-binding domains (Rrp4/cep1 synthetic lethality (Csl)4) is positioned on top of the hexamer on the opposite side to the RNA degrading sites. Here, we present the 1.6 A resolution crystal structure of the nine-subunit exosome of Sulfolobus solfataricus and the 2.3 A structure of this complex bound to an RNA substrate designed to be partly trimmed rather than completely degraded. The RNA binds both at the active site on one side of the molecule and on the opposite side in the narrowest constriction of the central channel. Multiple substrate-binding sites and the entrapment of the substrate in the central channel provide a rationale for the processive degradation of extended RNAs and the stalling of structured RNAs.