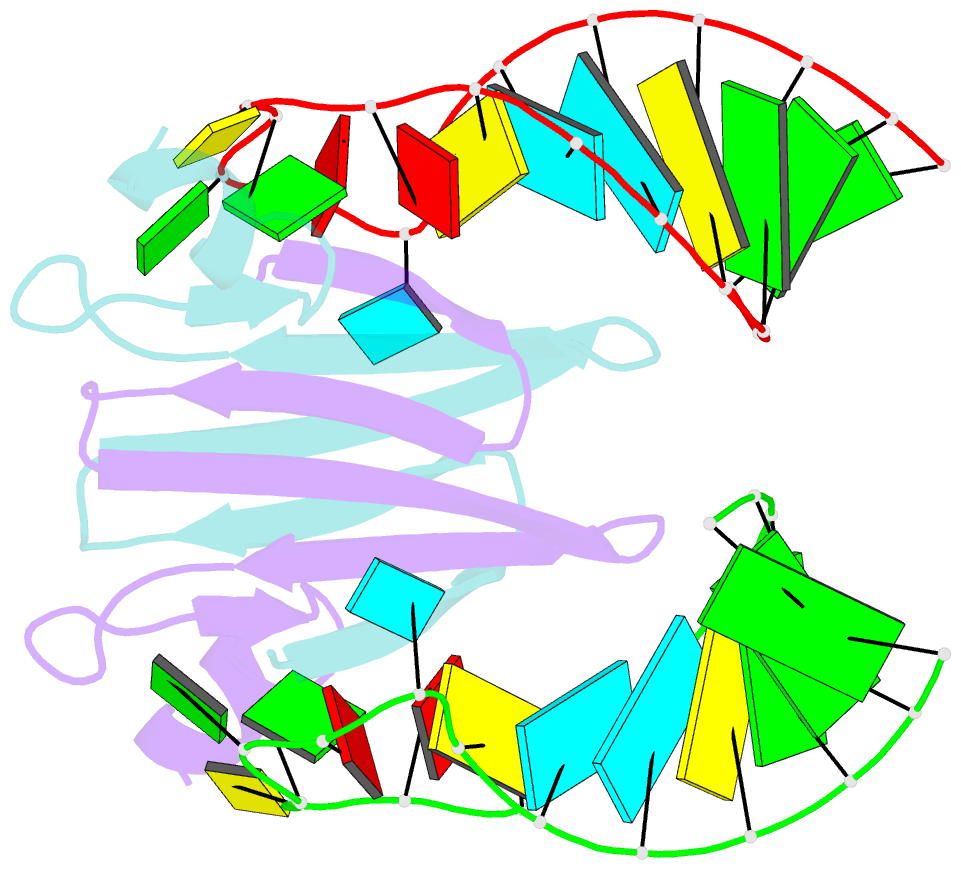
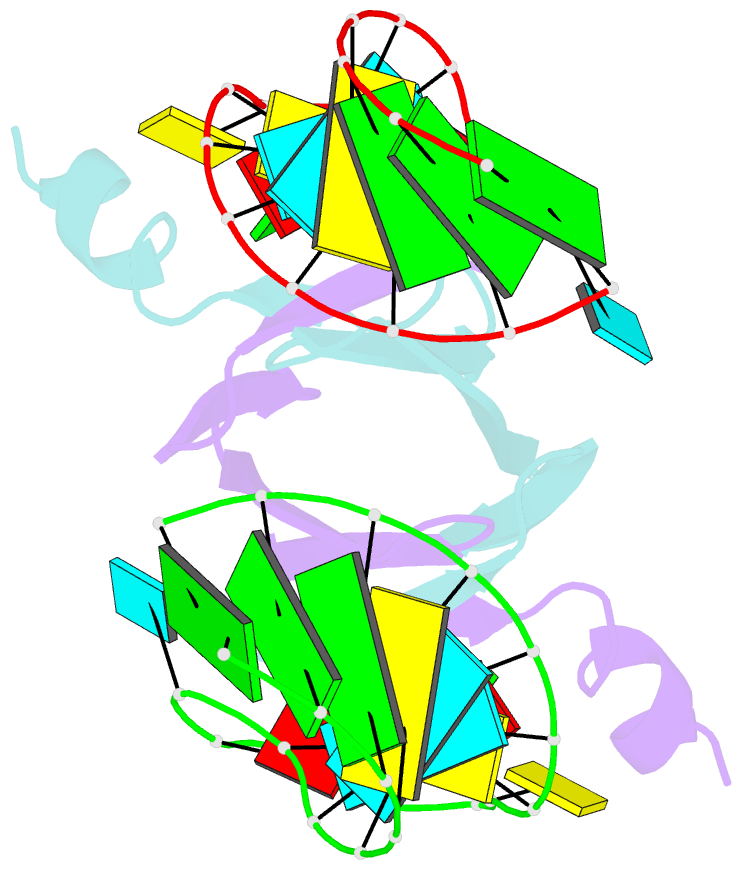
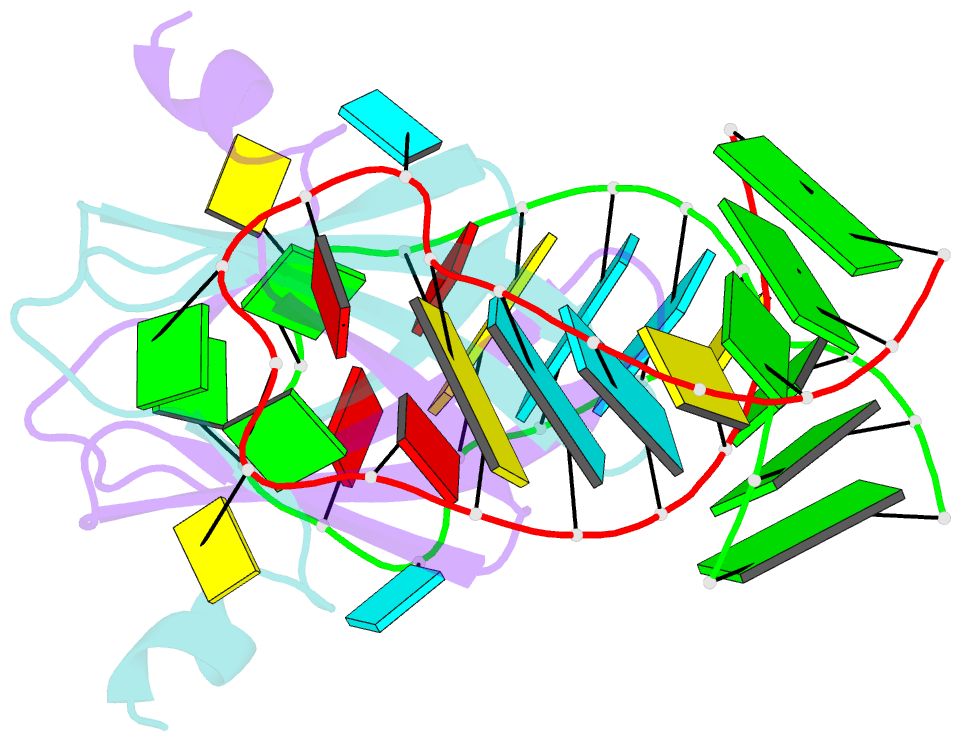
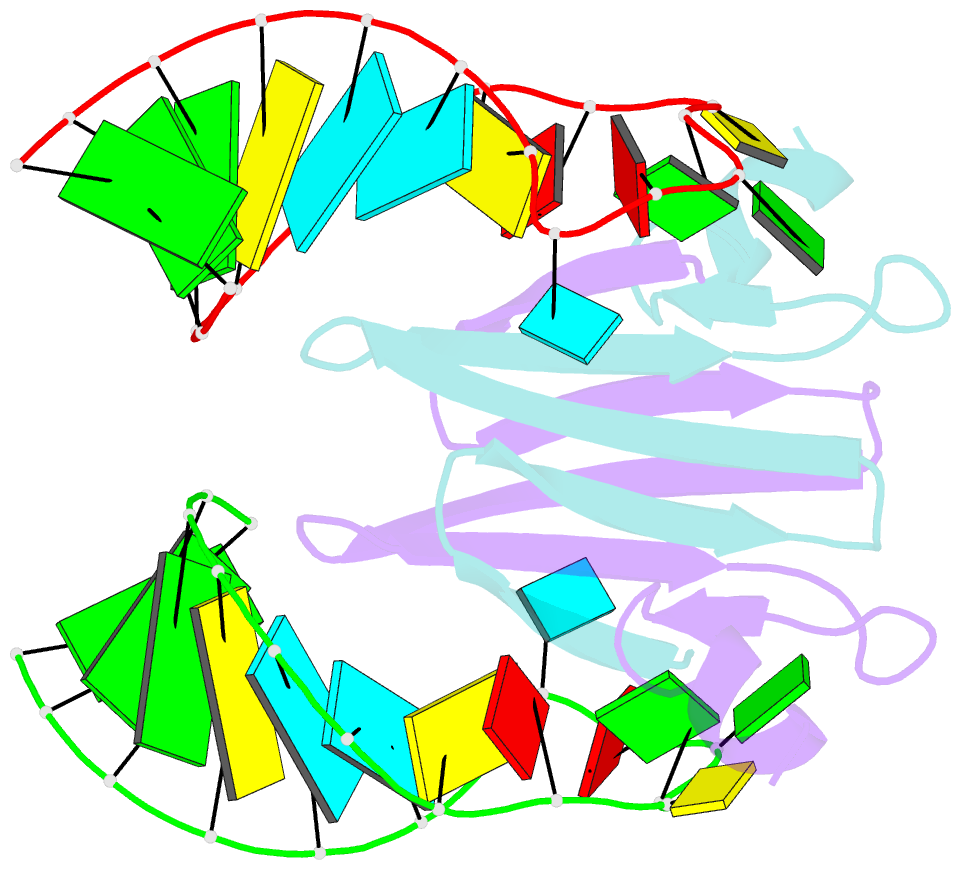
Summary information and primary citation
- PDB-id
- 2jpp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation-RNA
- Method
- NMR
- Summary
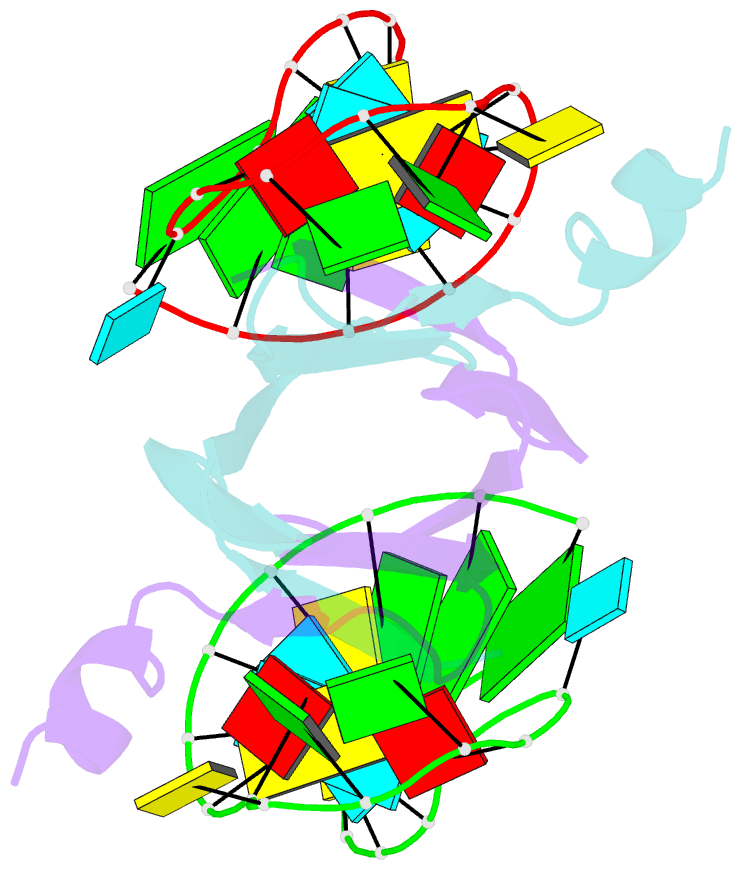
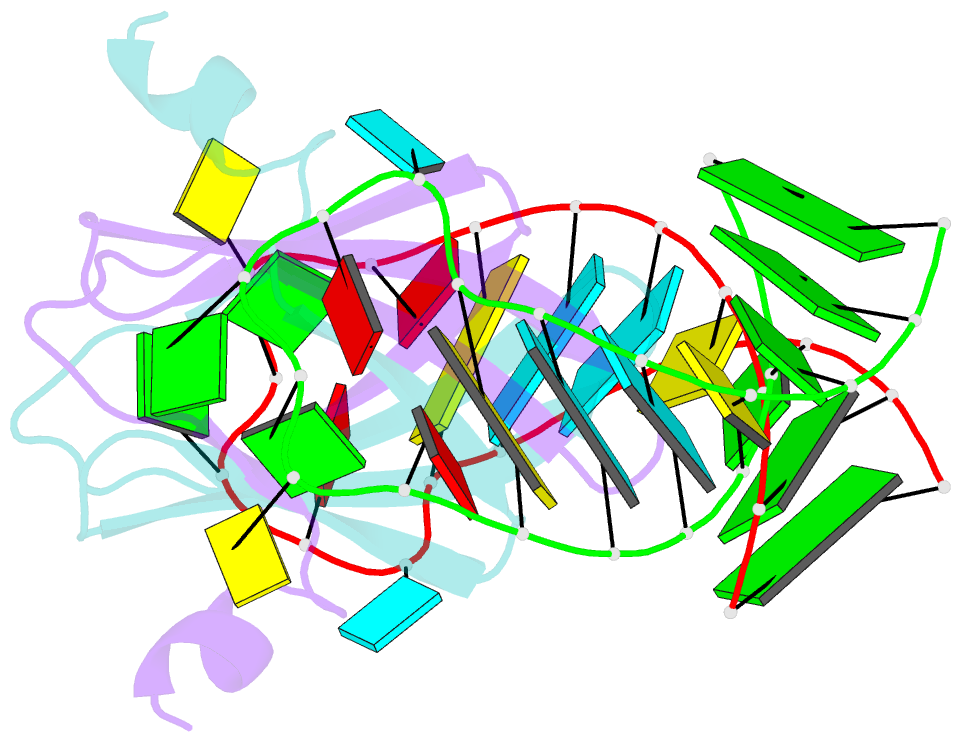
- Structural basis of rsma-csra RNA recognition: structure of rsme bound to the shine-dalgarno sequence of hcna mrna
- Reference
- Schubert M, Lapouge K, Duss O, Oberstrass FC, Jelesarov I, Haas D, Allain FH-T (2007): "Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA." Nat.Struct.Mol.Biol., 14, 807-813. doi: 10.1038/nsmb1285.
- Abstract
- Proteins of the RsmA/CsrA family are global translational regulators in many bacterial species. We have determined the solution structure of a complex formed between the RsmE protein, a member of this family from Pseudomonas fluorescens, and a target RNA encompassing the ribosome-binding site of the hcnA gene. The RsmE homodimer with its two RNA-binding sites makes optimal contact with an 5'-A/UCANGGANGU/A-3' sequence in the mRNA. When tightly gripped by RsmE, the ANGGAN core folds into a loop, favoring the formation of a 3-base-pair stem by flanking nucleotides. We validated these findings by in vivo and in vitro mutational analyses. The structure of the complex explains well how, by sequestering the Shine-Dalgarno sequence, the RsmA/CsrA proteins repress translation.