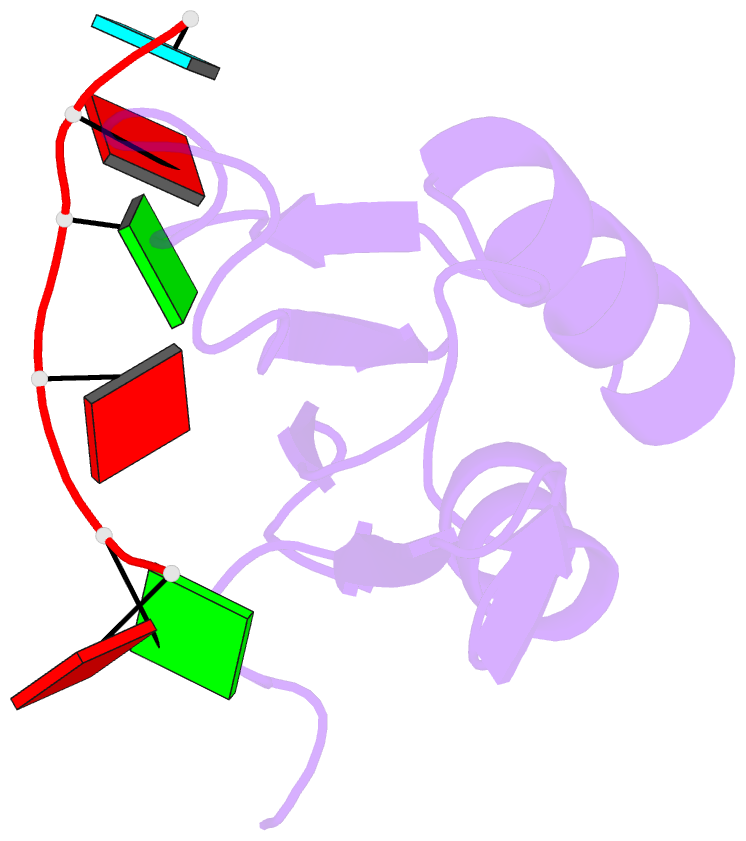
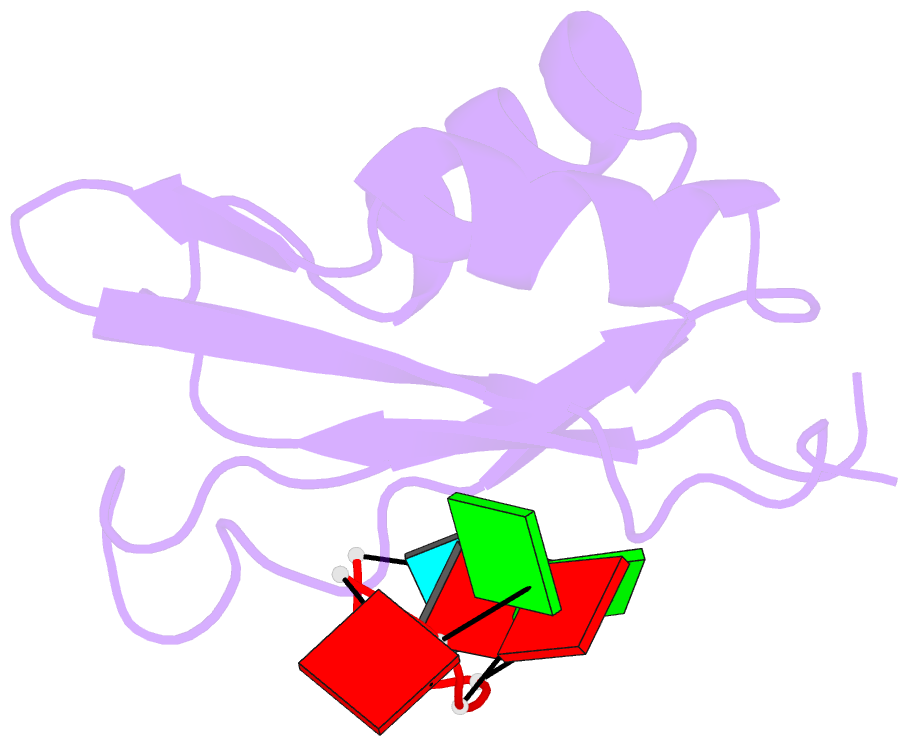
Summary information and primary citation
- PDB-id
- 2kh9; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- splicing-RNA
- Method
- NMR
- Summary
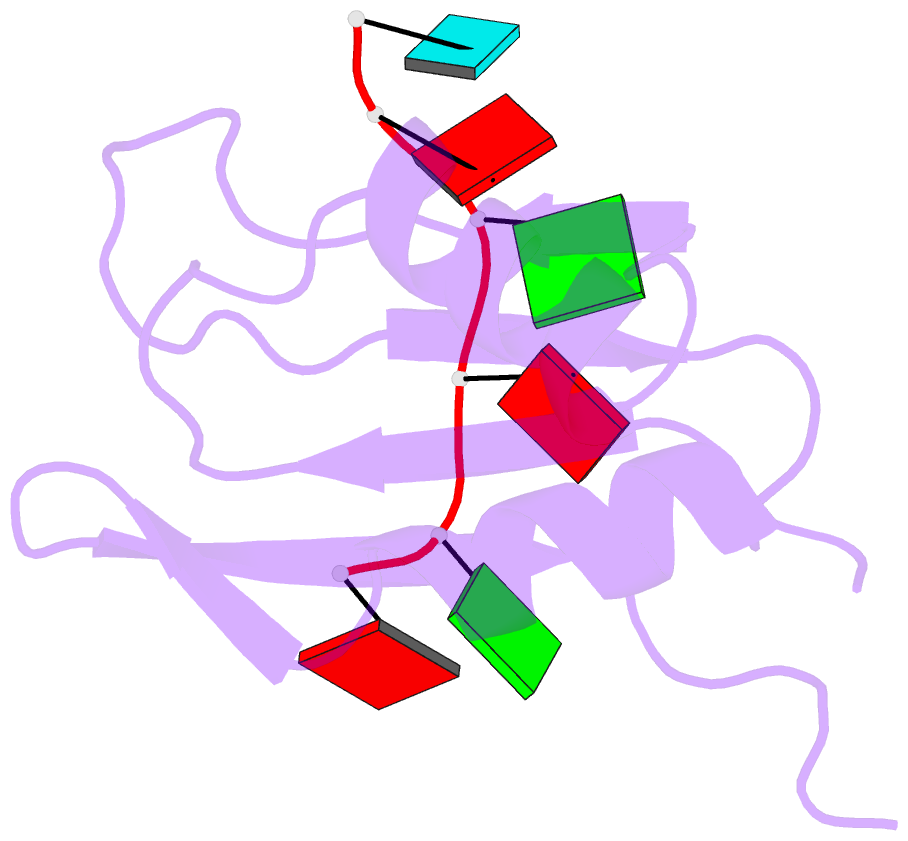
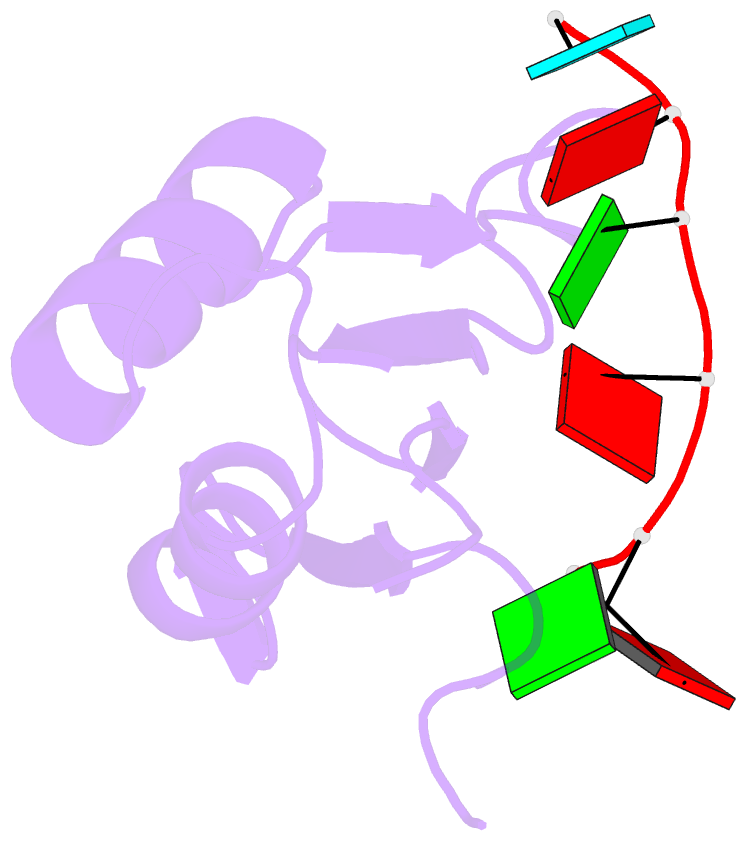
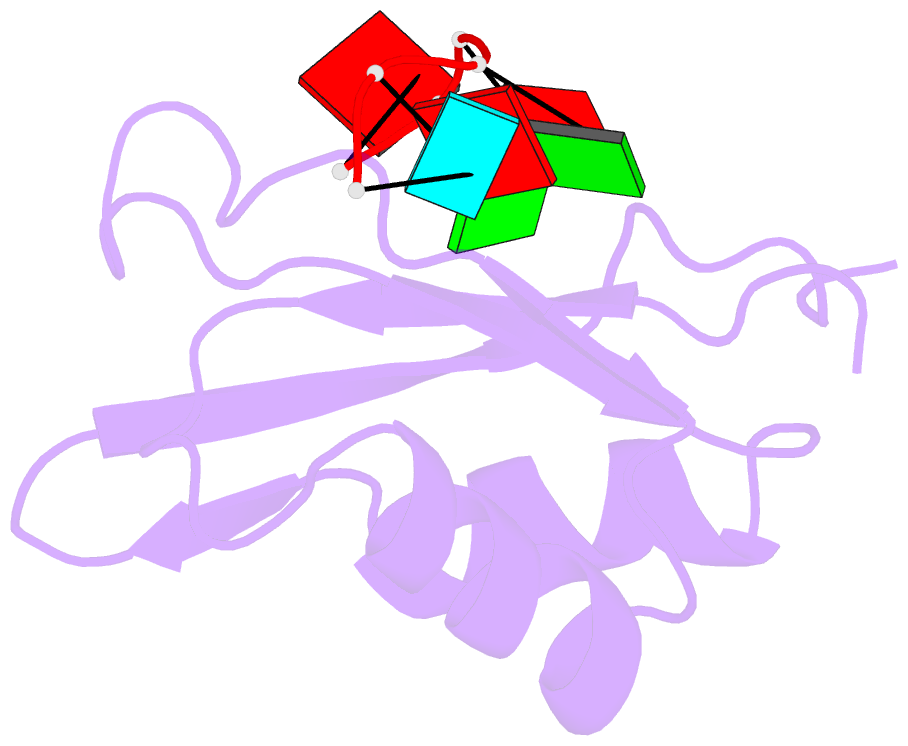
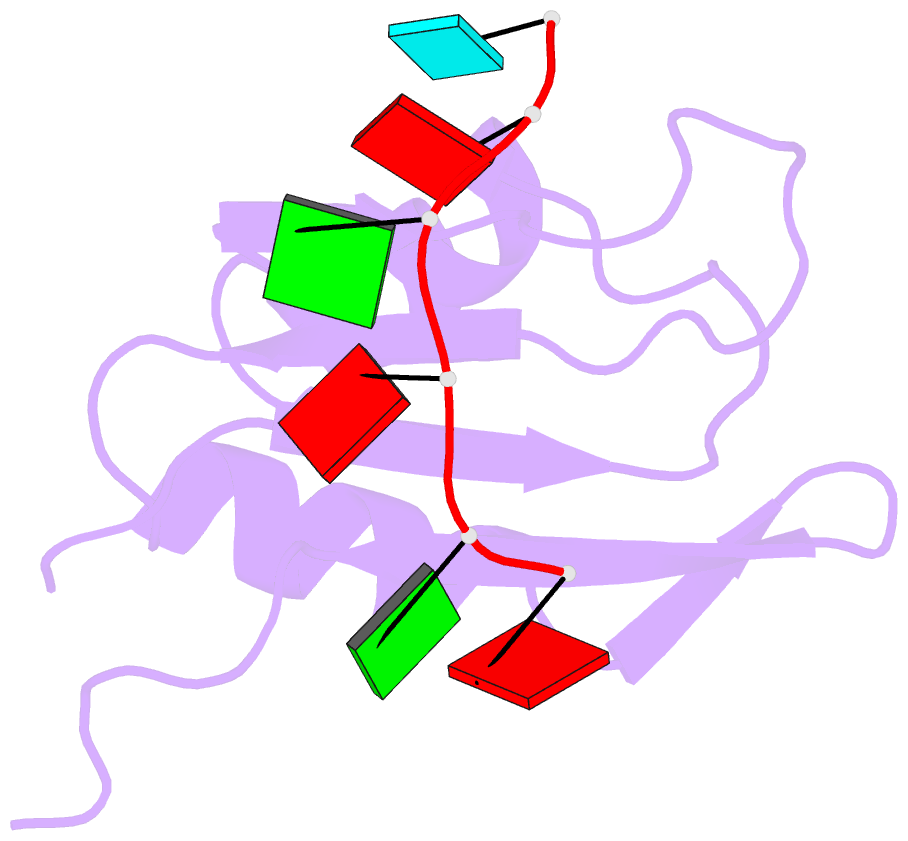
- Solution structure of yeast prp24-rrm2 bound to a fragment of u6 RNA
- Reference
- Martin-Tumasz S, Reiter NJ, Brow DA, Butcher SE (2010): "Structure and functional implications of a complex containing a segment of U6 RNA bound by a domain of Prp24." Rna, 16, 792-804. doi: 10.1261/rna.1913310.
- Abstract
- U6 RNA plays a critical role in pre-mRNA splicing. Assembly of U6 into the spliceosome requires a significant structural rearrangement and base-pairing with U4 RNA. In the yeast Saccharomyces cerevisiae, this process requires the essential splicing factor Prp24. We present the characterization and structure of a complex containing one of Prp24's four RNA recognition motif (RRM) domains, RRM2, and a fragment of U6 RNA. NMR methods were used to identify the preferred U6 binding sequence of RRM2 (5'-GAGA-3'), measure the affinity of the interaction, and solve the structure of RRM2 bound to the hexaribonucleotide AGAGAU. Interdomain contacts observed between RRM2 and RRM3 in a crystal structure of the free protein are not detectable in solution. A structural model of RRM1 and RRM2 bound to a longer segment of U6 RNA is presented, and a partial mechanism for Prp24's annealing activity is proposed.