Summary information and primary citation
- PDB-id
- 2kxn; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- NMR
- Summary
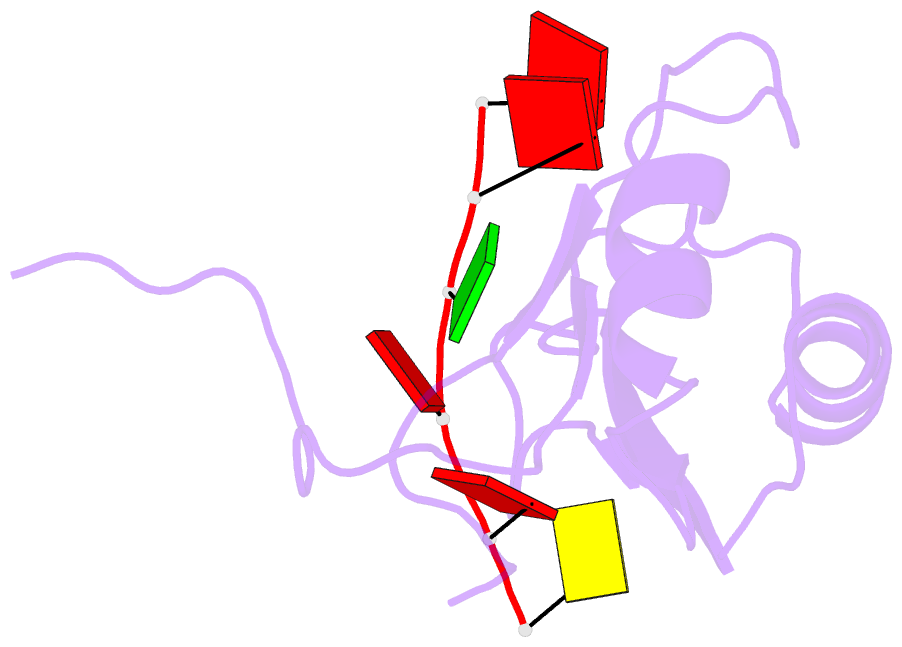
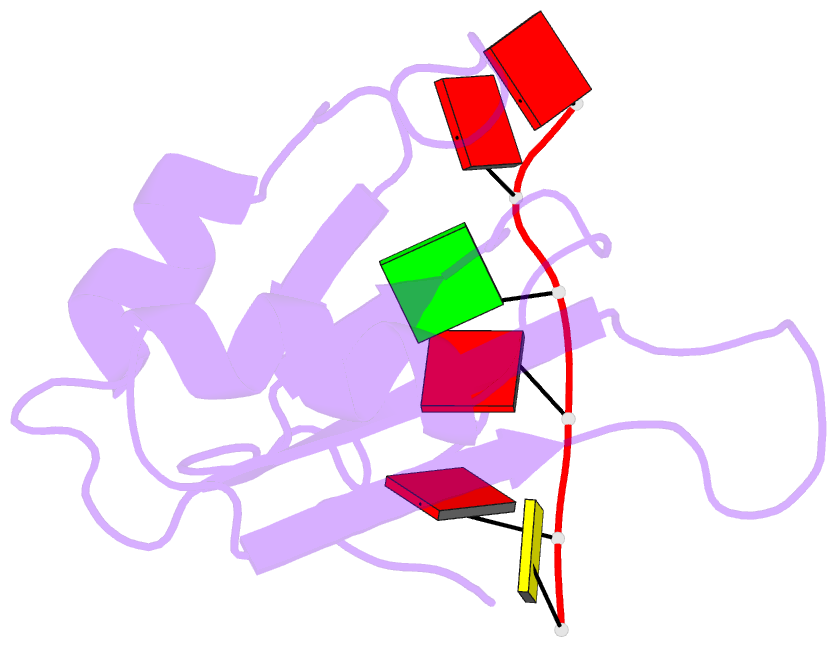
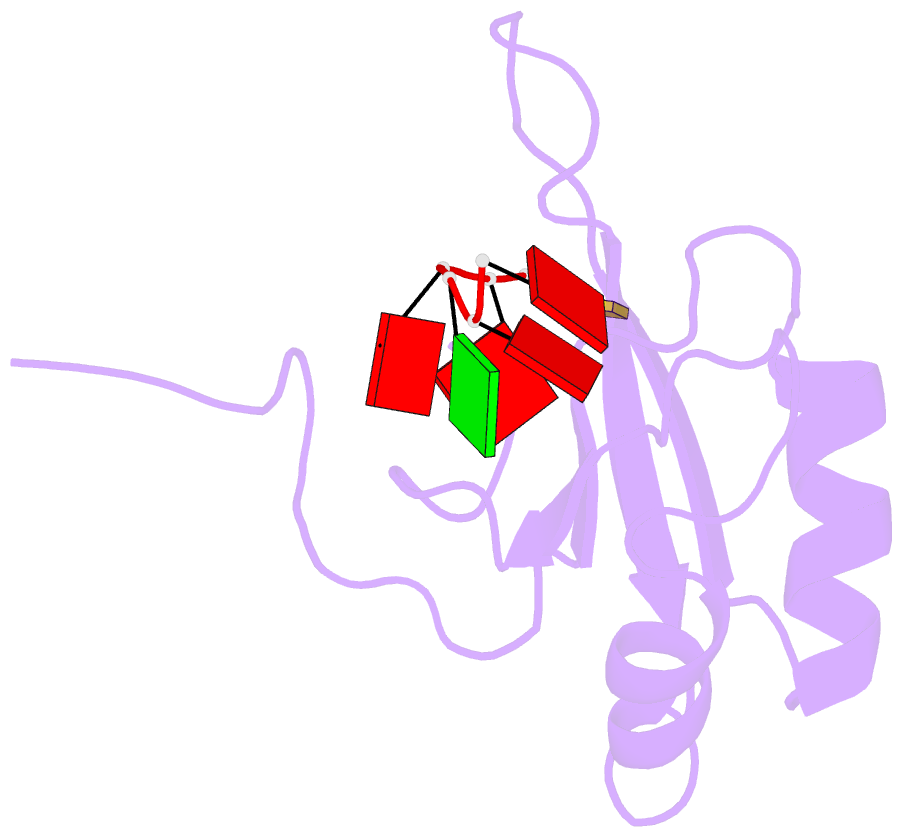
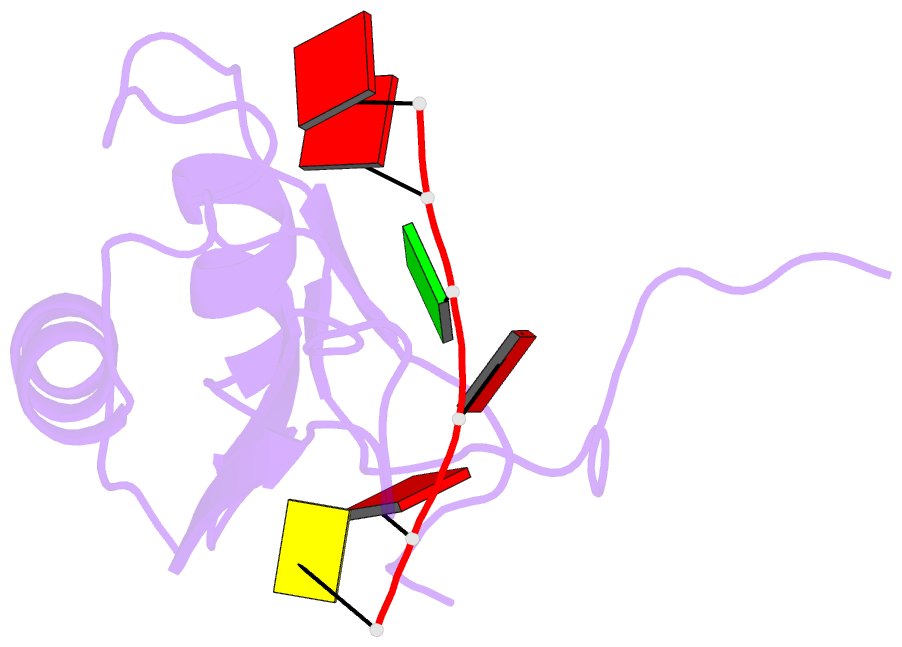
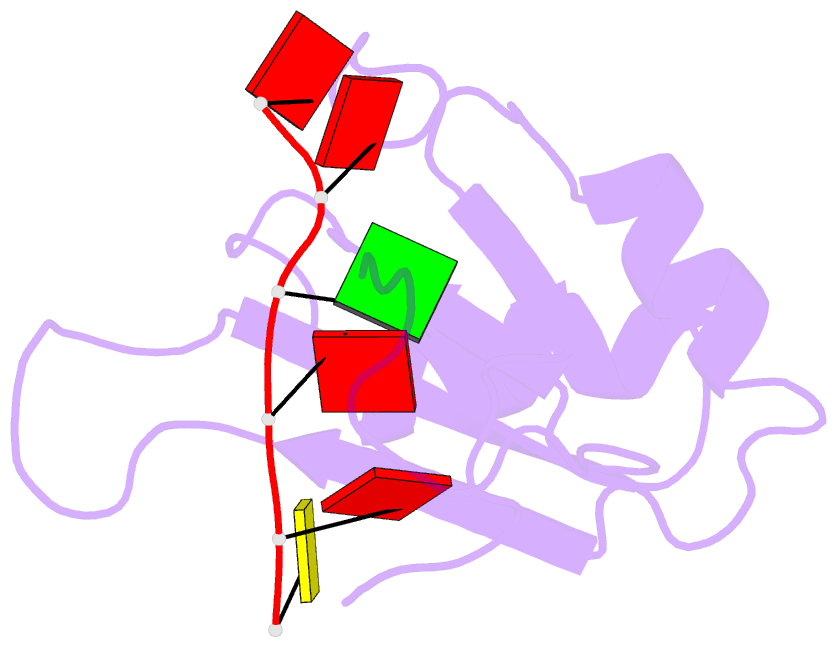
- NMR structure of human tra2beta1 rrm in complex with aagaac RNA
- Reference
- Clery A, Jayne S, Benderska N, Dominguez C, Stamm S, Allain FH (2011): "Molecular basis of purine-rich RNA recognition by the human SR-like protein Tra2-beta1." Nat.Struct.Mol.Biol., 18, 443-450. doi: 10.1038/nsmb.2001.
- Abstract
- Tra2-β1 is a unique splicing factor as its single RNA recognition motif (RRM) is located between two RS (arginine-serine) domains. To understand how this protein recognizes its RNA target, we solved the structure of Tra2-β1 RRM in complex with RNA. The central 5'-AGAA-3' motif is specifically recognized by residues from the β-sheet of the RRM and by residues from both extremities flanking the RRM. The structure suggests that RNA binding by Tra2-β1 induces positioning of the two RS domains relative to one another. By testing the effect of Tra2-β1 and RNA mutations on the splicing of SMN2 exon 7, we validated the importance of the RNA-protein contacts observed in the structure for the function of Tra2-β1 and determined the functional sequence of Tra2-β1 in SMN2 exon 7. Finally, we propose a model for the assembly of multiple RNA binding proteins on this exon.