Summary information and primary citation
- PDB-id
- 2mf1; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- NMR
- Summary
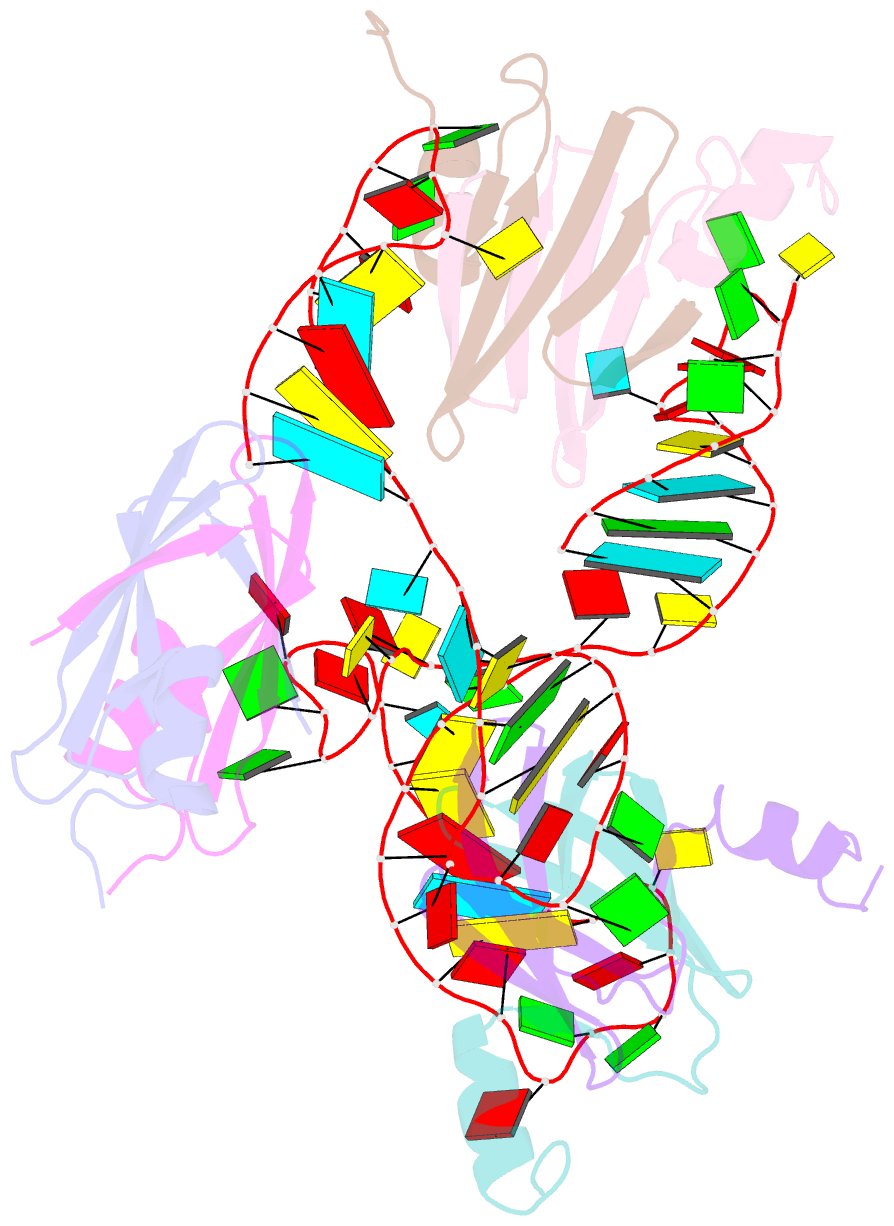
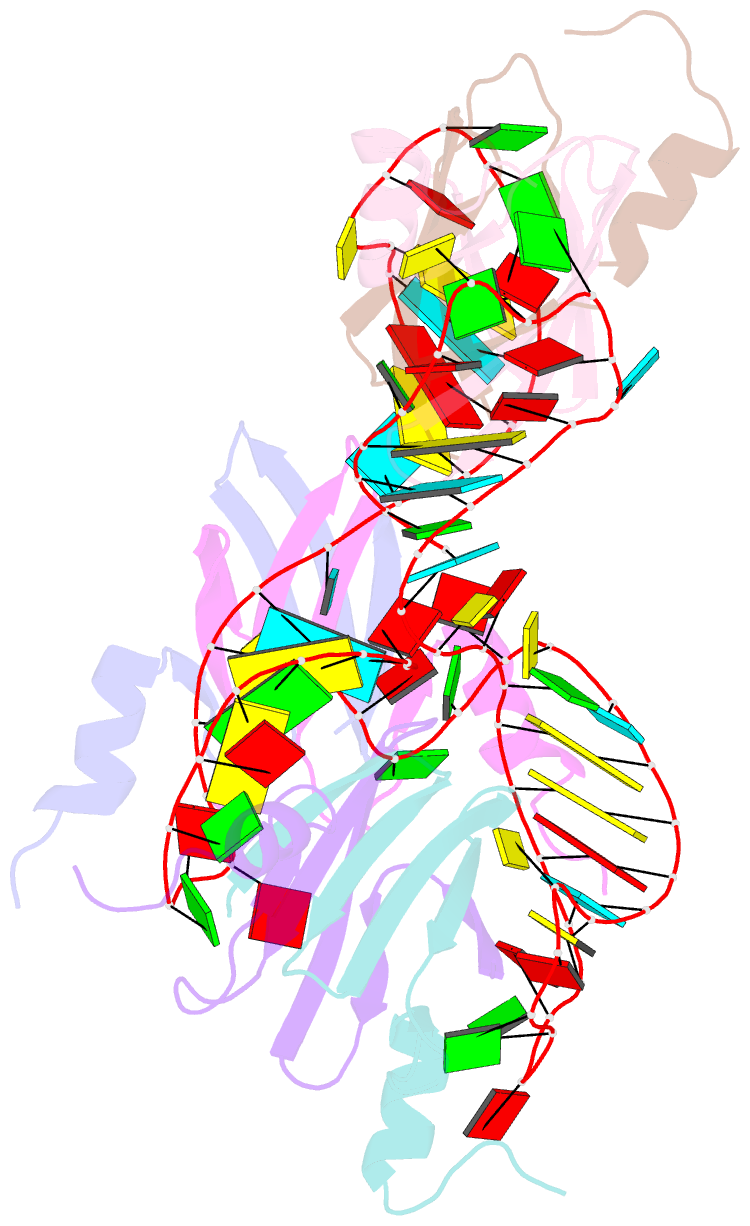
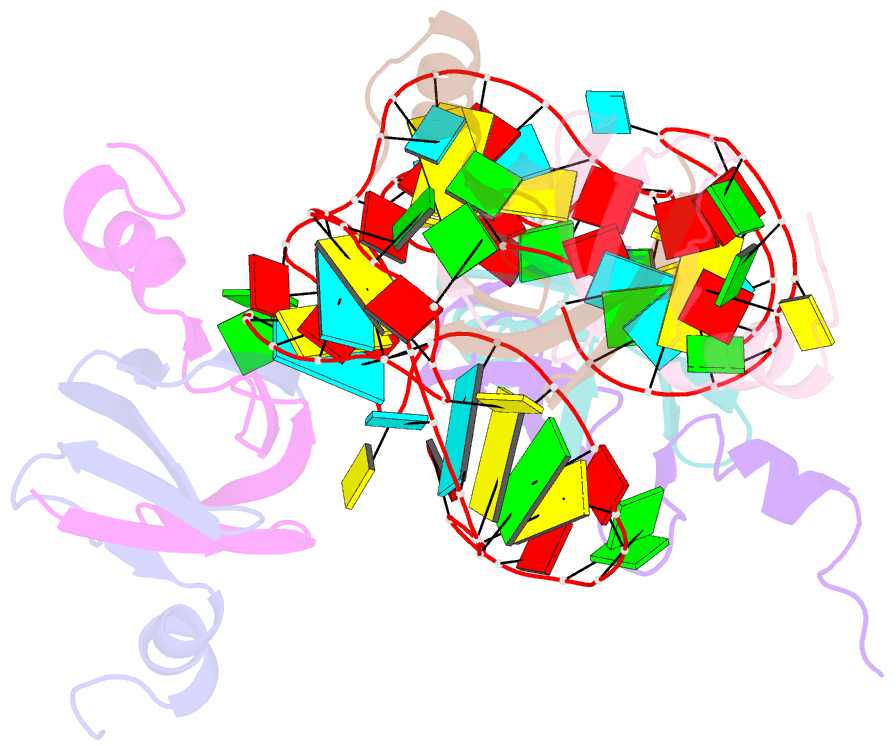
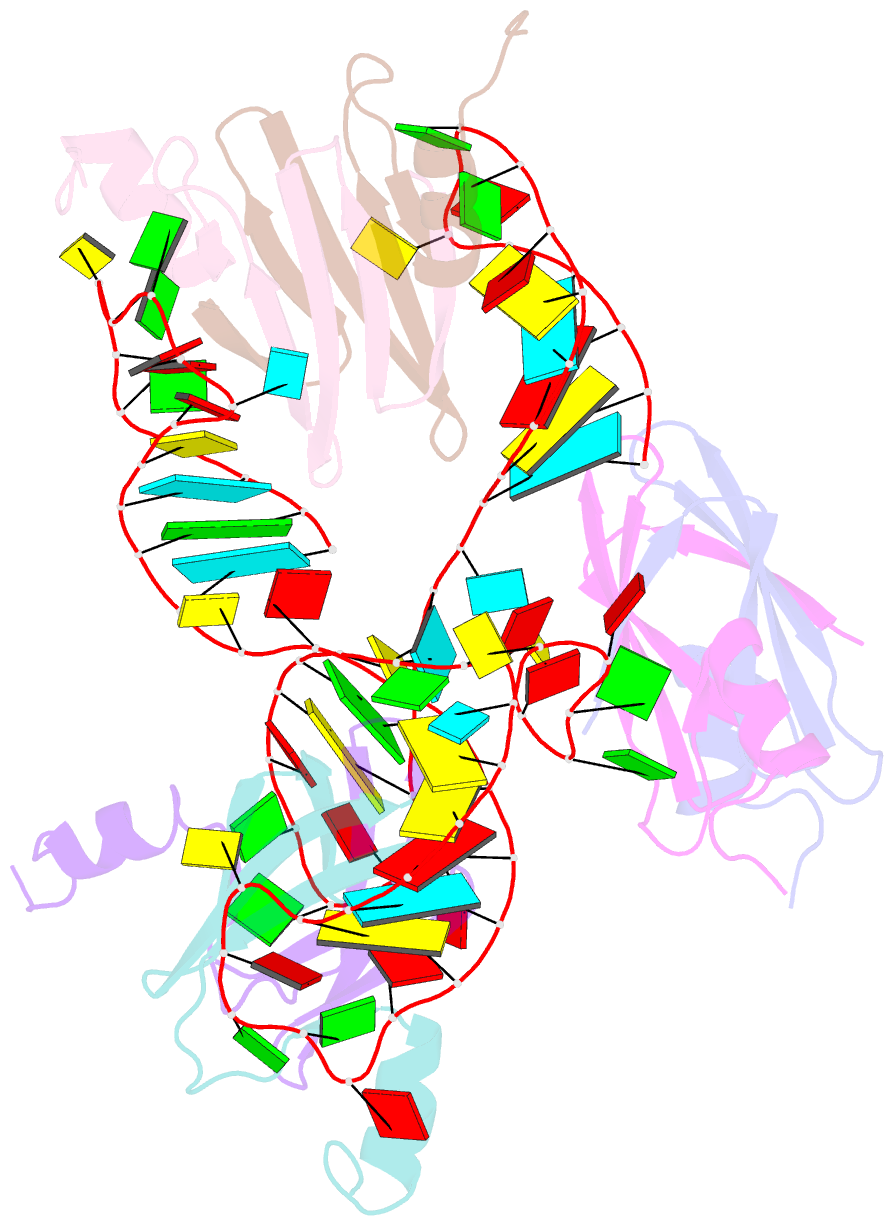
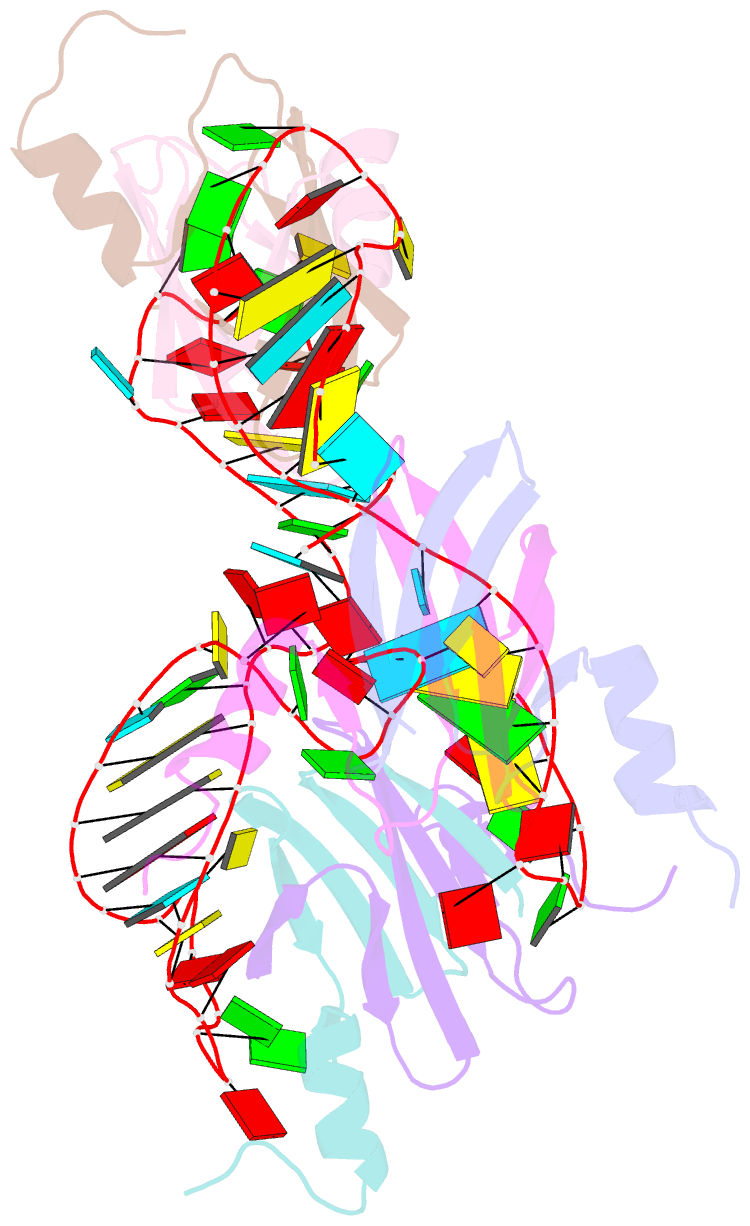
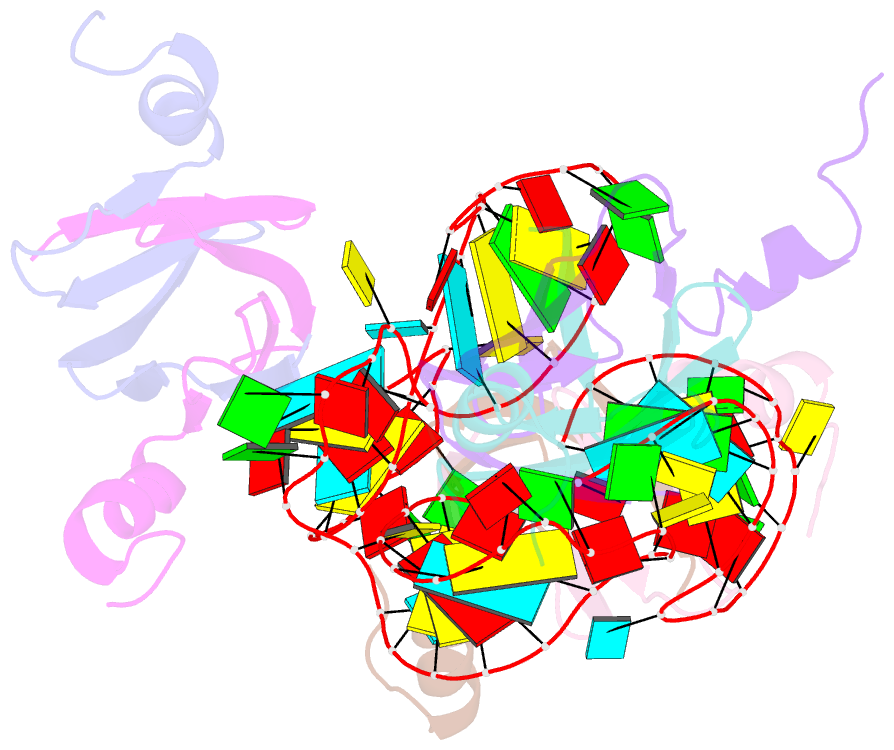
- Structural basis of the non-coding RNA rsmz acting as protein sponge: conformer r of rsmz(1-72)-rsme(dimer) 1to3 complex
- Reference
- Duss O, Michel E, Yulikov M, Schubert M, Jeschke G, Allain FH (2014): "Structural basis of the non-coding RNA RsmZ acting as a protein sponge." Nature, 509, 588-592. doi: 10.1038/nature13271.
- Abstract
- MicroRNA and protein sequestration by non-coding RNAs (ncRNAs) has recently generated much interest. In the bacterial Csr/Rsm system, which is considered to be the most general global post-transcriptional regulatory system responsible for bacterial virulence, ncRNAs such as CsrB or RsmZ activate translation initiation by sequestering homodimeric CsrA-type proteins from the ribosome-binding site of a subset of messenger RNAs. However, the mechanism of ncRNA-mediated protein sequestration is not understood at the molecular level. Here we show for Pseudomonas fluorescens that RsmE protein dimers assemble sequentially, specifically and cooperatively onto the ncRNA RsmZ within a narrow affinity range. This assembly yields two different native ribonucleoprotein structures. Using a powerful combination of nuclear magnetic resonance and electron paramagnetic resonance spectroscopy we elucidate these 70-kilodalton solution structures, thereby revealing the molecular mechanism of the sequestration process and how RsmE binding protects the ncRNA from RNase E degradation. Overall, our findings suggest that RsmZ is well-tuned to sequester, store and release RsmE and therefore can be viewed as an ideal protein 'sponge'.