Summary information and primary citation
- PDB-id
- 2n21; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- NMR
- Summary
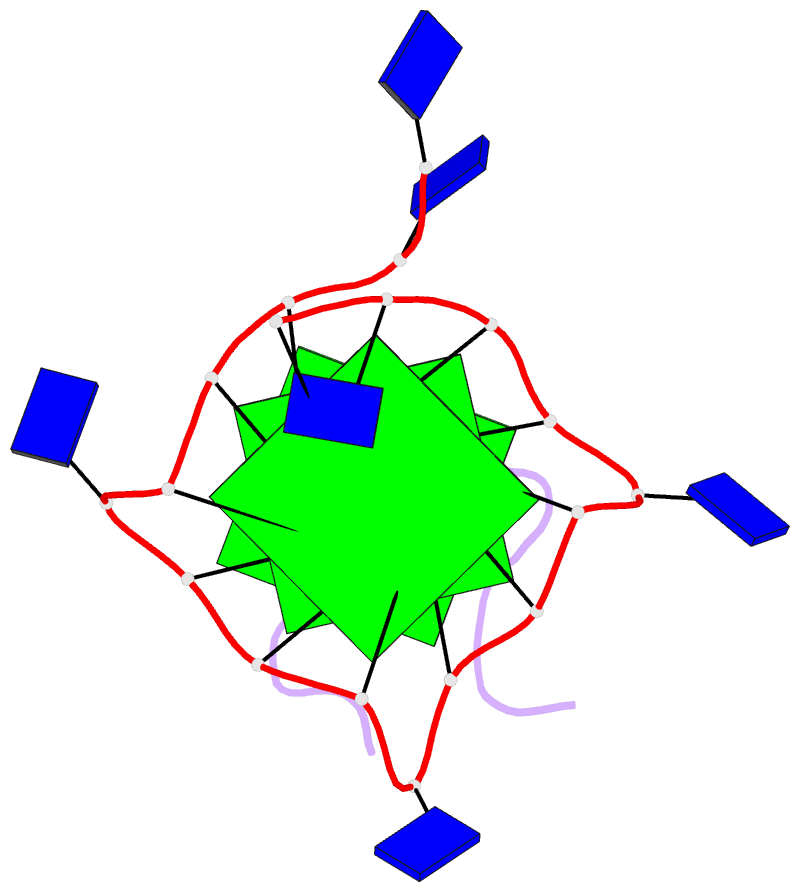
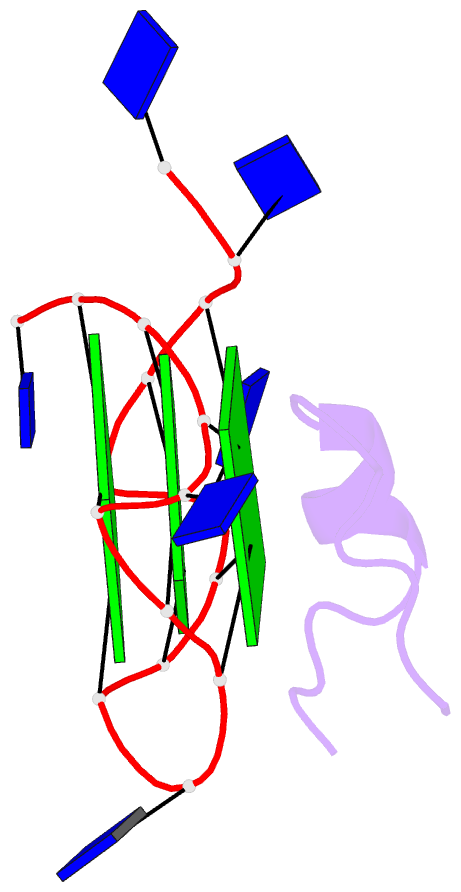
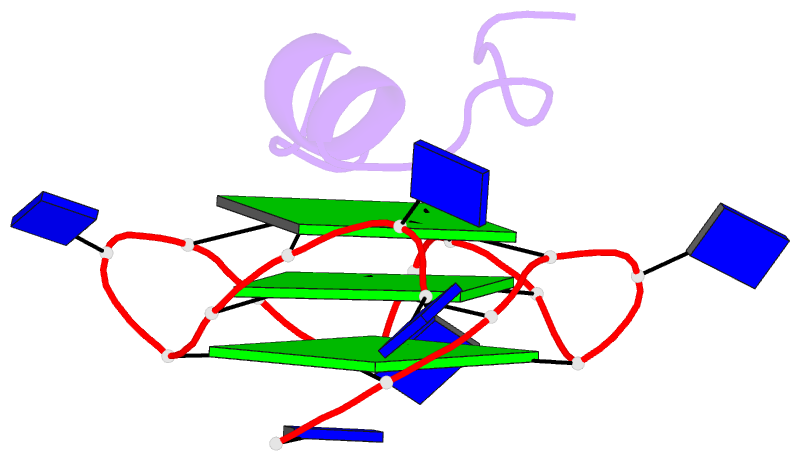
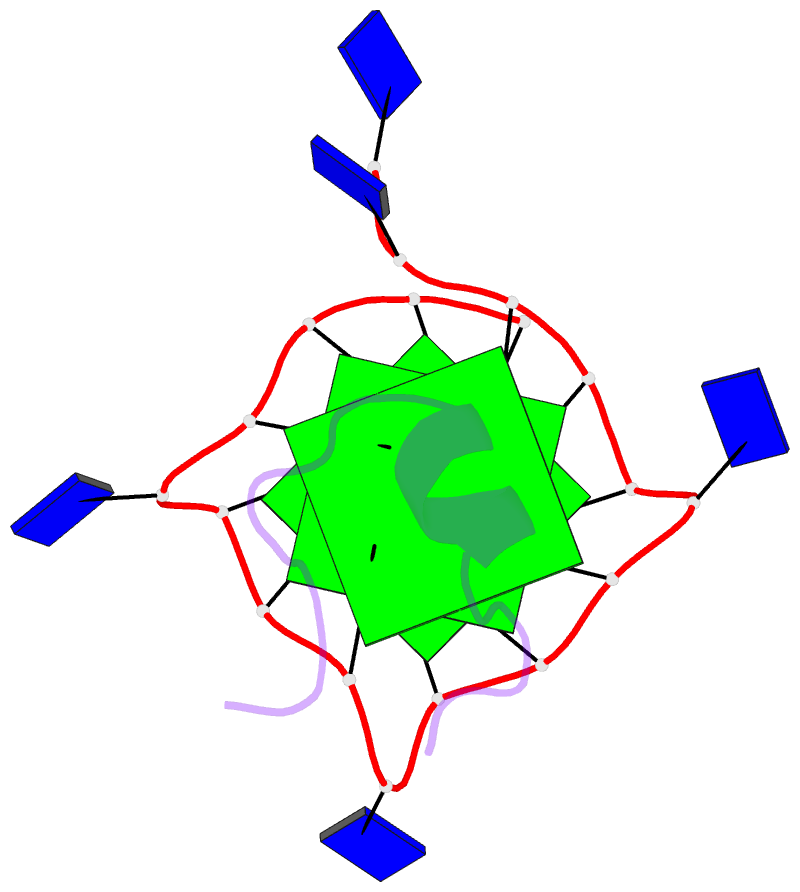
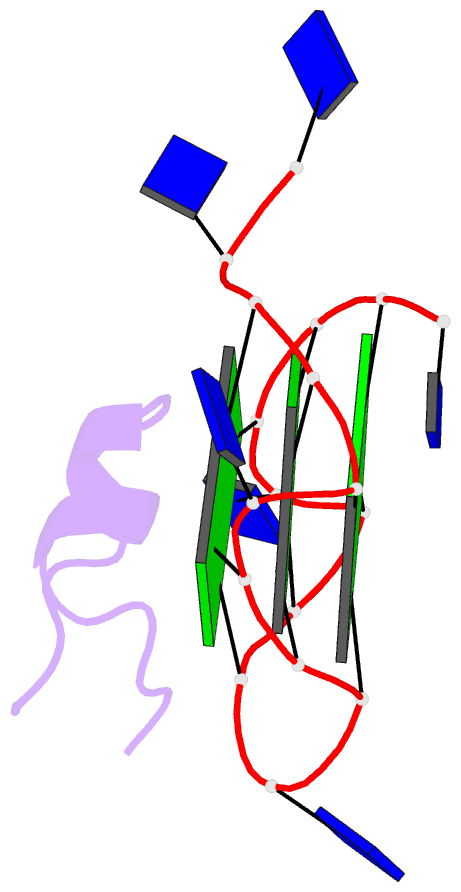
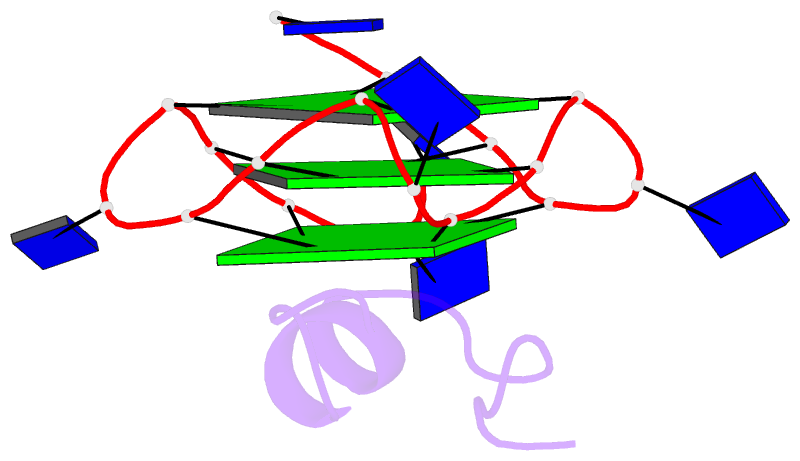
- Solution structure of complex between DNA g-quadruplex and g-quadruplex recognition domain of rhau
- Reference
- Heddi B, Cheong VV, Martadinata H, Phan AT (2015): "Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: Solution structure of a peptide-quadruplex complex." Proc.Natl.Acad.Sci.USA, 112, 9608-9613. doi: 10.1073/pnas.1422605112.
- Abstract
- Four-stranded nucleic acid structures called G-quadruplexes have been associated with important cellular processes, which should require G-quadruplex-protein interaction. However, the structural basis for specific G-quadruplex recognition by proteins has not been understood. The DEAH (Asp-Glu-Ala-His) box RNA helicase associated with AU-rich element (RHAU) (also named DHX36 or G4R1) specifically binds to and resolves parallel-stranded G-quadruplexes. Here we identified an 18-amino acid G-quadruplex-binding domain of RHAU and determined the structure of this peptide bound to a parallel DNA G-quadruplex. Our structure explains how RHAU specifically recognizes parallel G-quadruplexes. The peptide covers a terminal guanine base tetrad (G-tetrad), and clamps the G-quadruplex using three-anchor-point electrostatic interactions between three positively charged amino acids and negatively charged phosphate groups. This binding mode is strikingly similar to that of most ligands selected for specific G-quadruplex targeting. Binding to an exposed G-tetrad represents a simple and efficient way to specifically target G-quadruplex structures.