Summary information and primary citation
- PDB-id
- 2nny; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.58 Å)
- Summary
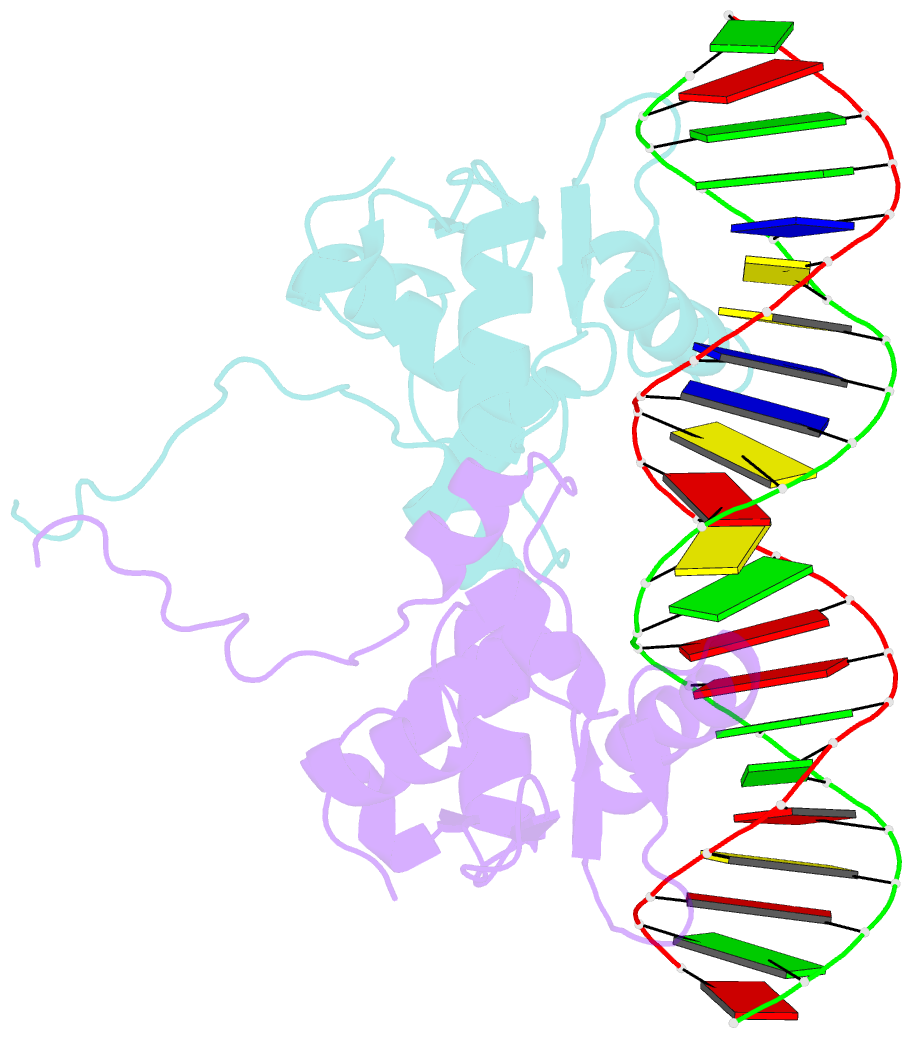
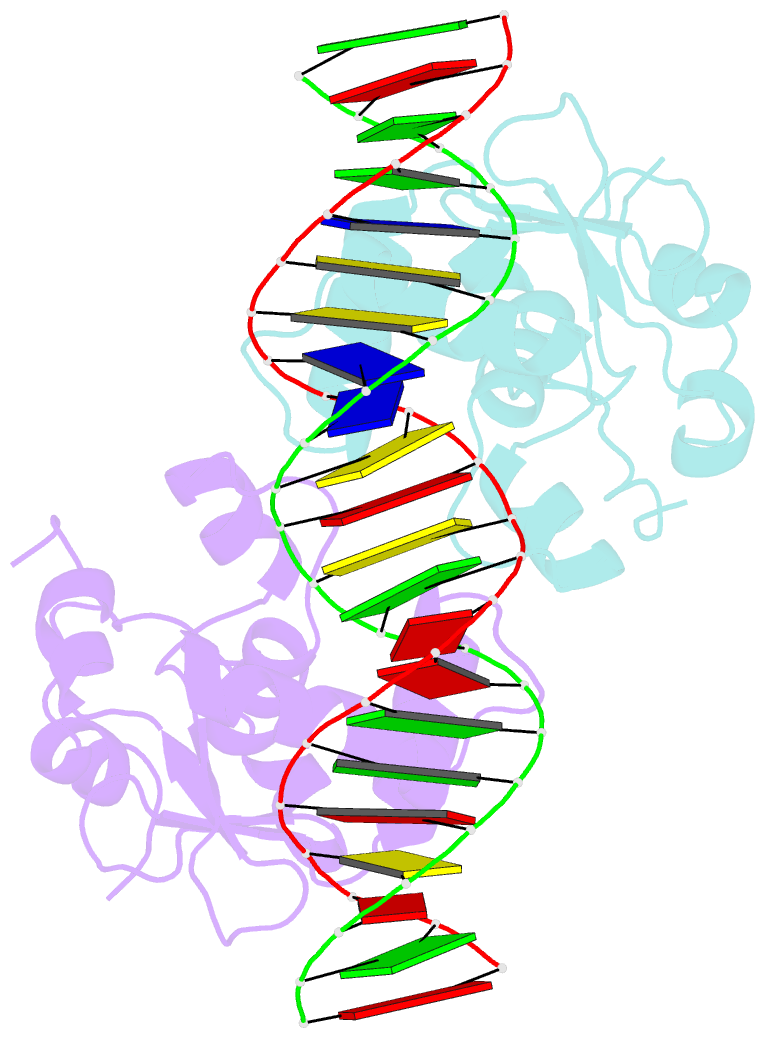
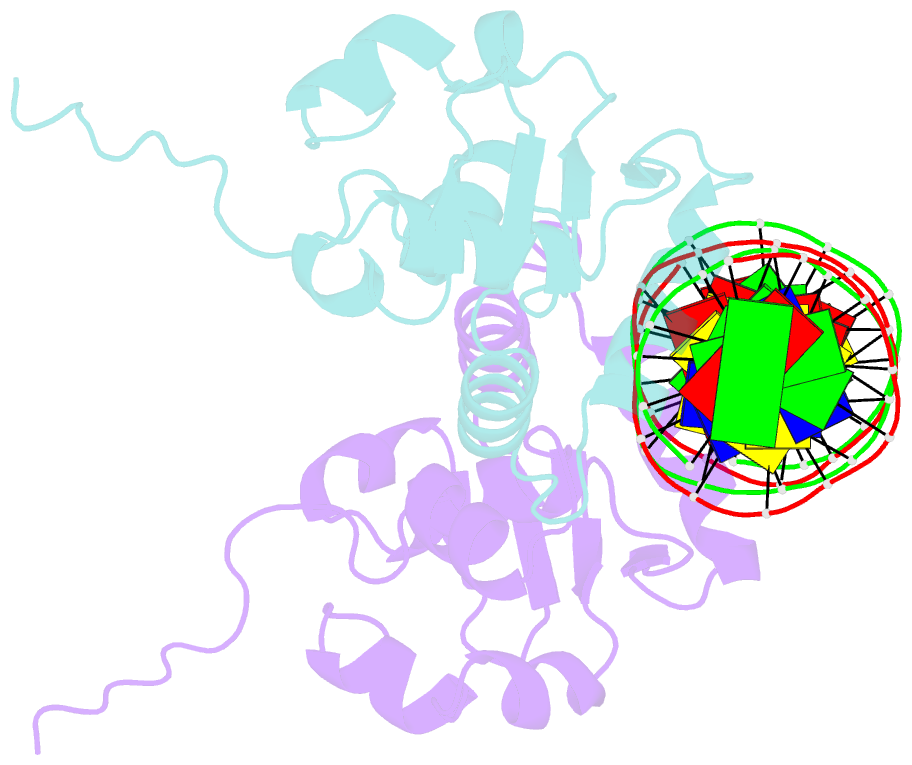
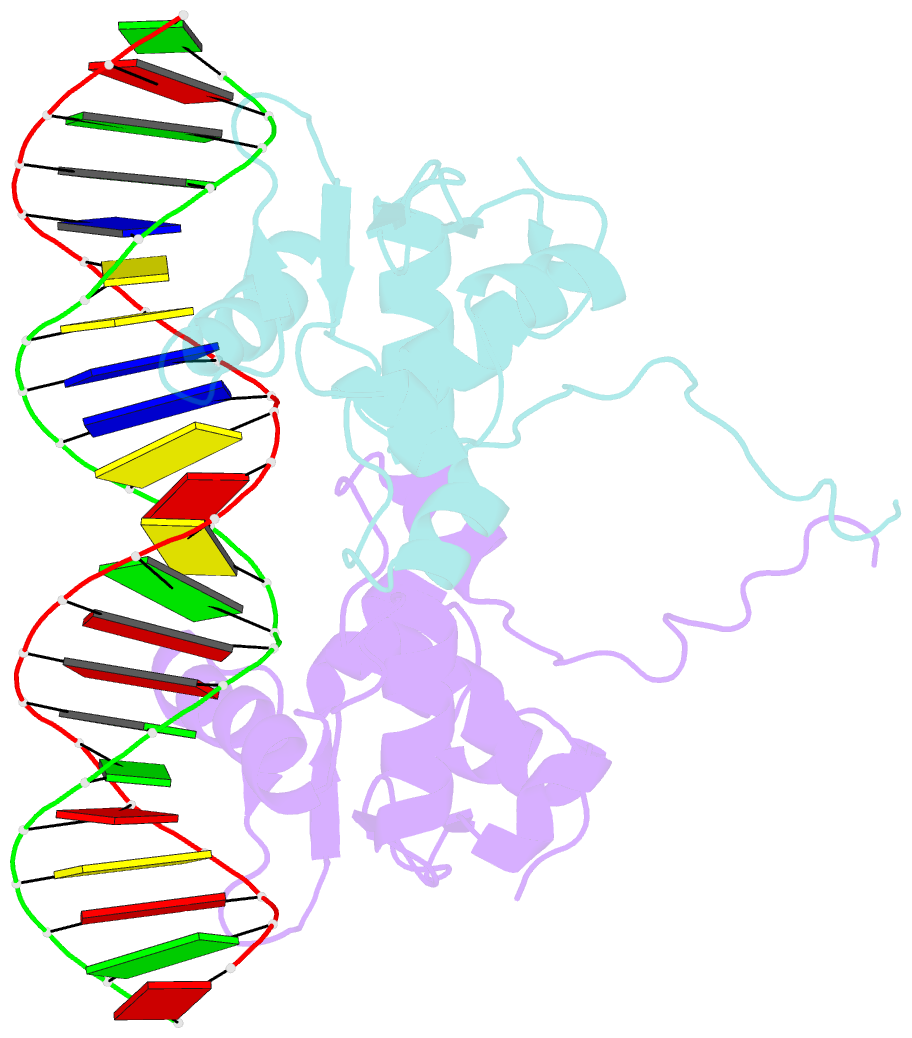
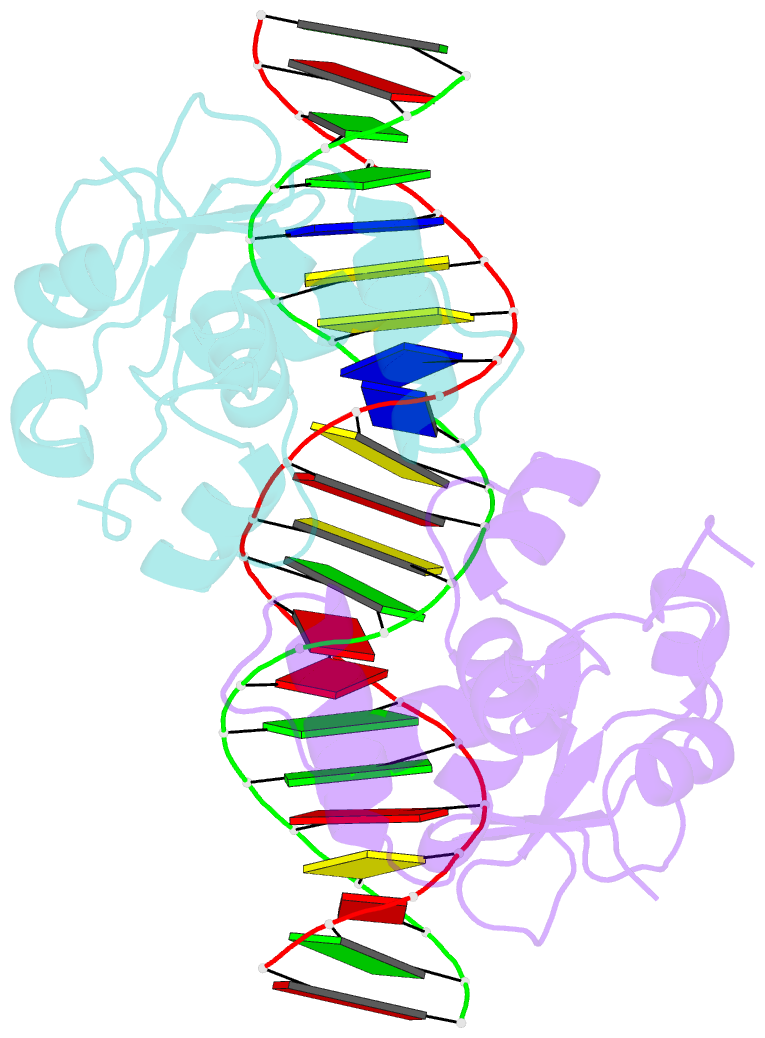
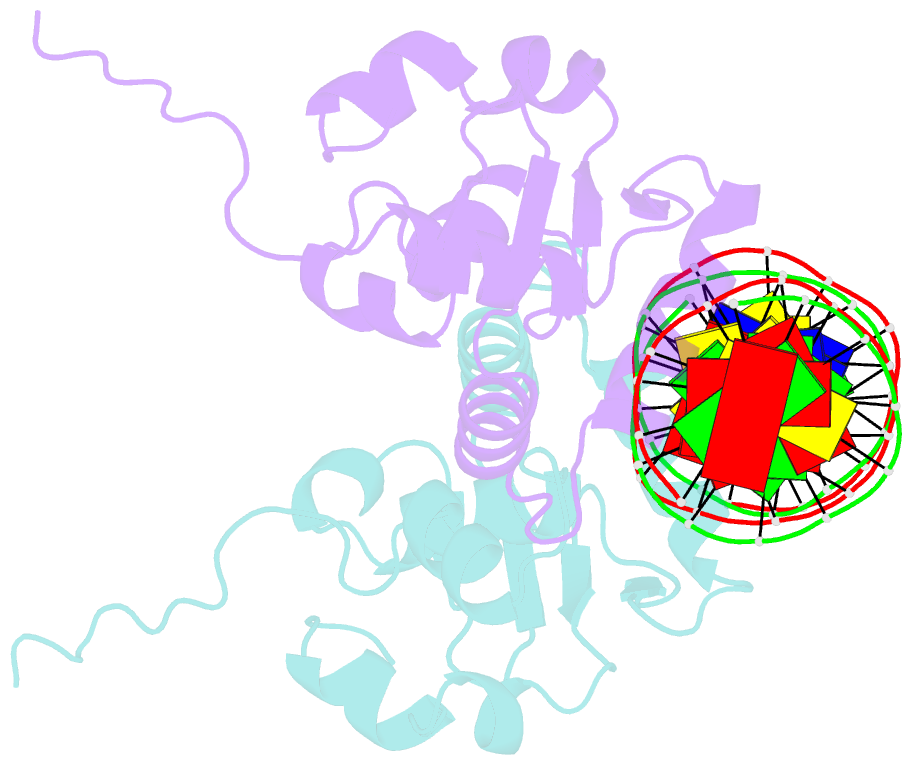
- Crystal structure of the ets1 dimer DNA complex.
- Reference
- Lamber EP, Vanhille L, Textor LC, Kachalova GS, Sieweke MH, Wilmanns M (2008): "Regulation of the transcription factor Ets-1 by DNA-mediated homo-dimerization." Embo J., 27, 2006-2017. doi: 10.1038/emboj.2008.117.
- Abstract
- The function of the Ets-1 transcription factor is regulated by two regions that flank its DNA-binding domain. A previously established mechanism for auto-inhibition of monomeric Ets-1 on DNA response elements with a single ETS-binding site, however, has not been observed for the stromelysin-1 promoter containing two palindromic ETS-binding sites. We present the structure of Ets-1 on this promoter element, revealing a ternary complex in which protein homo-dimerization is mediated by the specific arrangement of the two ETS-binding sites. In this complex, the N-terminal-flanking region is required for ternary protein-DNA assembly. Ets-1 variants, in which residues from this region are mutated, loose the ability for DNA-mediated dimerization and stromelysin-1 promoter transactivation. Thus, our data unravel the molecular basis for relief of auto-inhibition and the ability of Ets-1 to function as a facultative dimeric transcription factor on this site. Our findings may also explain previous data of Ets-1 function in the context of heterologous transcription factors, thus providing a molecular model that could also be valid for Ets-1 regulation by hetero-oligomeric assembly.