Summary information and primary citation
- PDB-id
- 2ntz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- cell cycle-DNA
- Method
- X-ray (3.35 Å)
- Summary
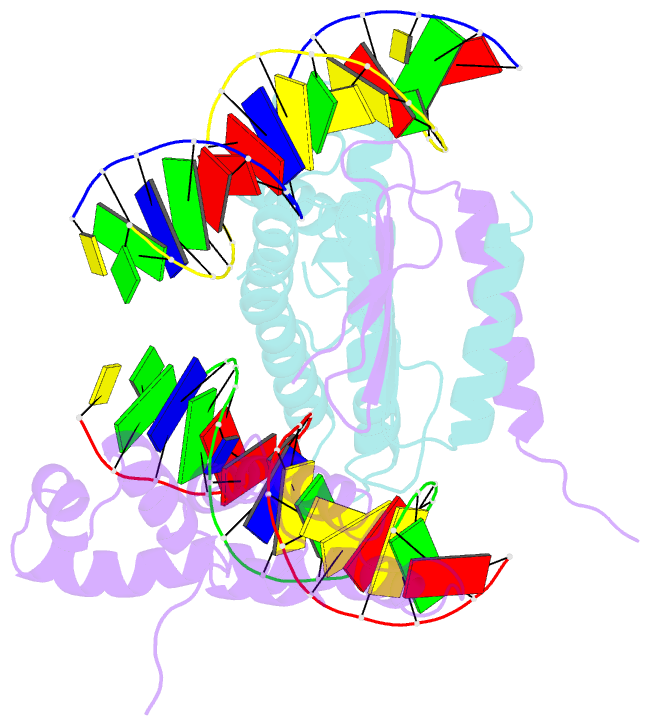
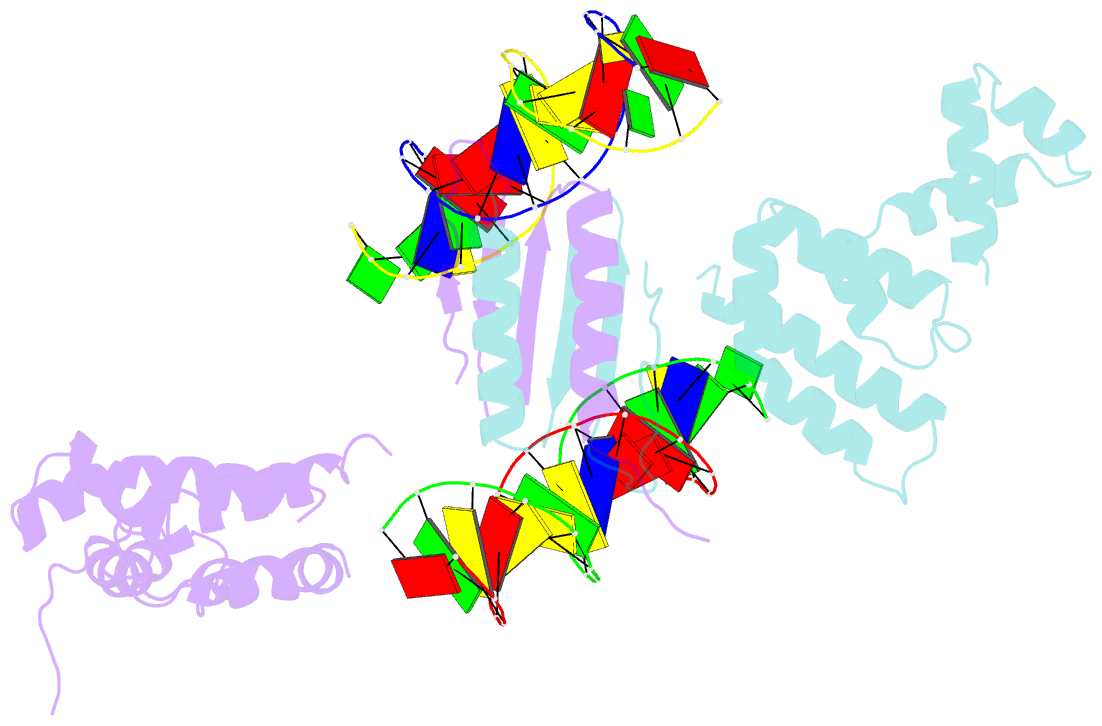
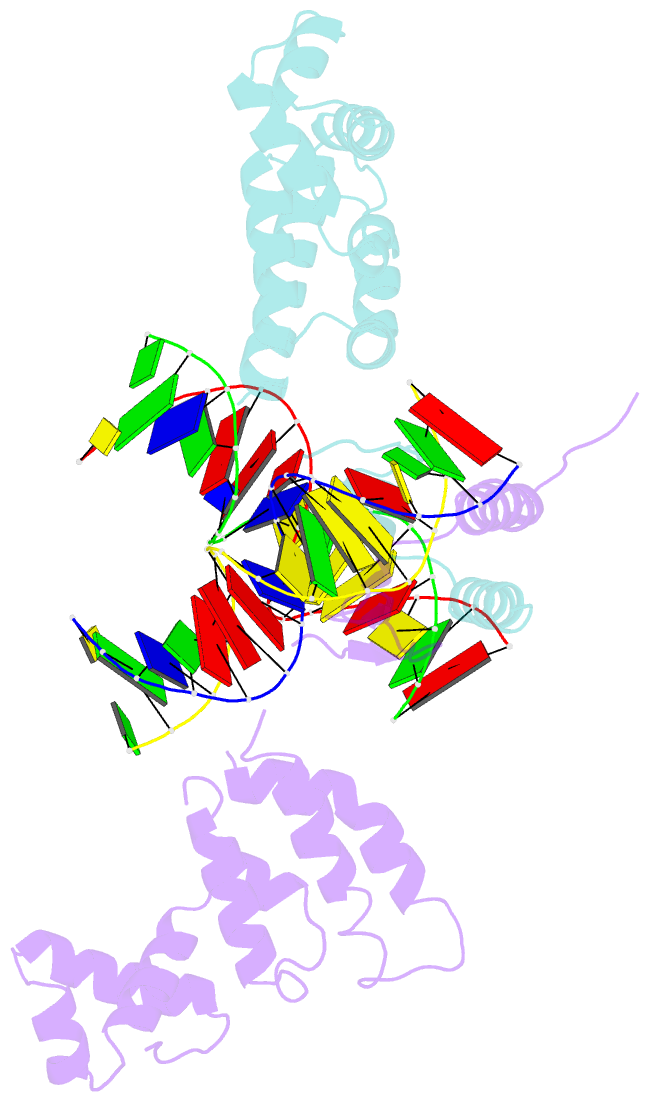
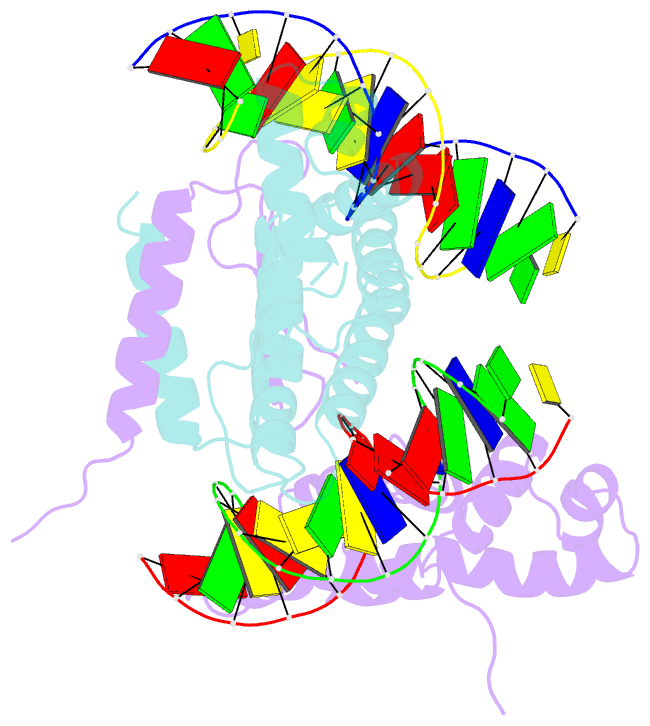
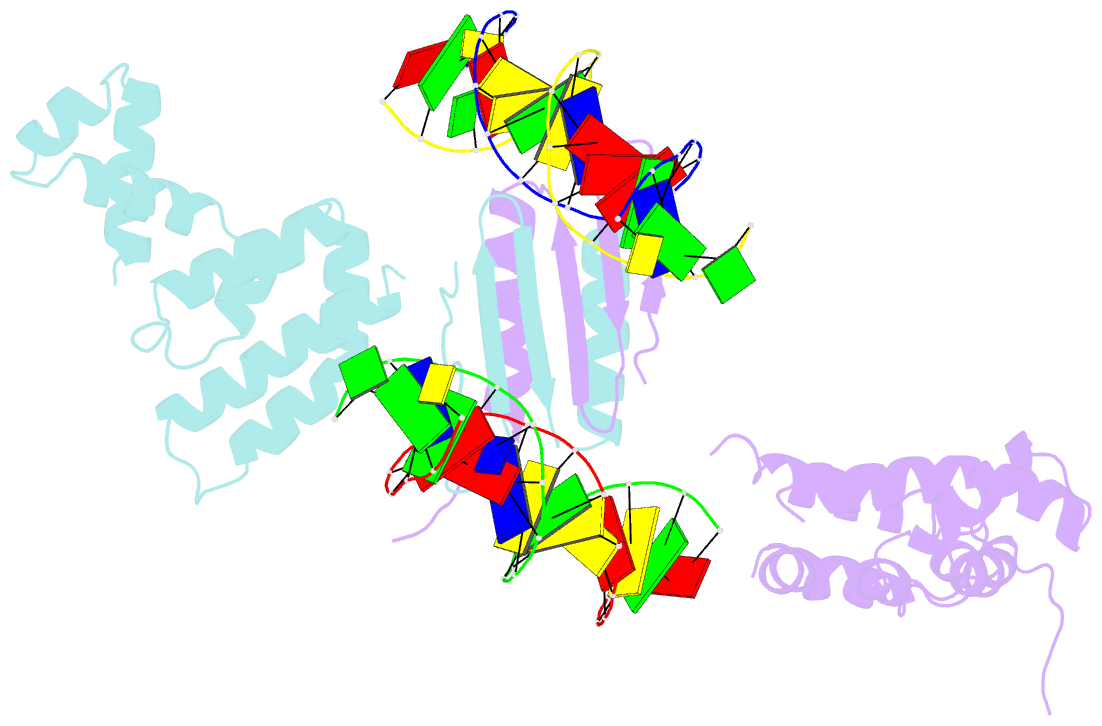
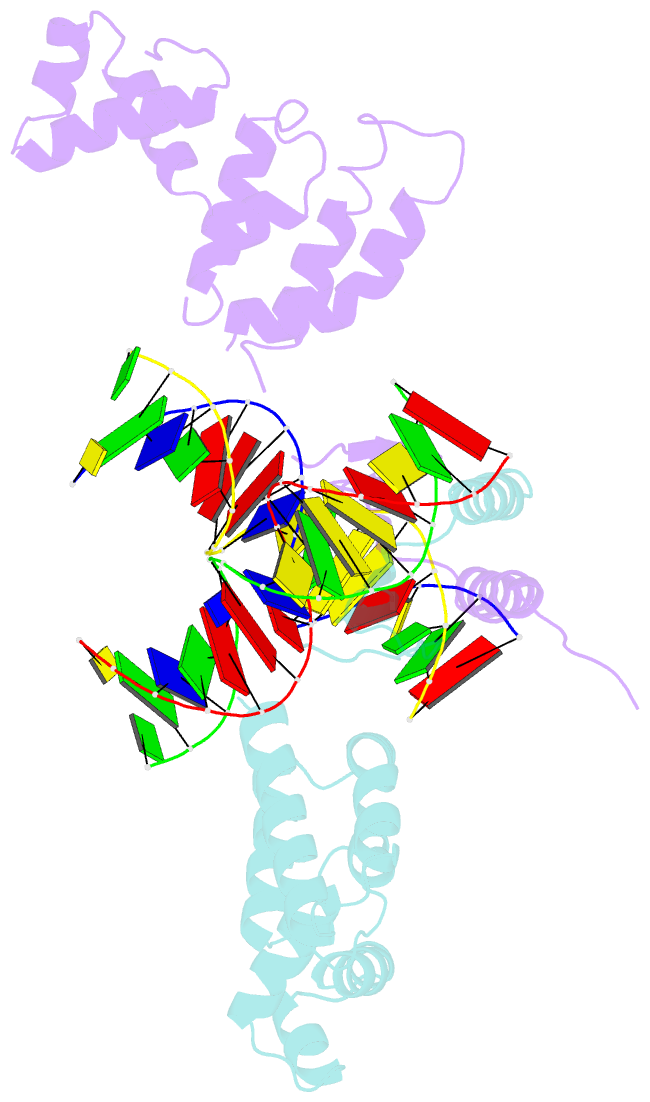
- Structure of a parb-DNA complex reveals a double b-box interaction
- Reference
- Schumacher MA, Mansoor A, Funnell BE (2007): "Structure of a four-way bridged ParB-DNA complex provides insight into P1 segrosome assembly." J.Biol.Chem., 282, 10456-10464. doi: 10.1074/jbc.M610603200.
- Abstract
- The plasmid partition process is essential for plasmid propagation and is mediated by par systems, consisting of centromere-like sites and two proteins, ParA and ParB. In the first step of partition by the archetypical P1 system, ParB binds a complicated centromere-like site to form a large nucleoprotein segrosome. ParB is a dimeric DNA-binding protein that can bridge between both A-boxes and B-boxes located on the centromere. Its helix-turn-helix domains bind A-boxes and the dimer domain binds B-boxes. Binding of the first ParB dimer nucleates the remaining ParB molecules onto the centromere site, which somehow leads to the formation of a condensed segrosome superstructure. To further understand this unique DNA spreading capability of ParB, we crystallized and determined the structure of a 1:2 ParB-(142-333):A3-B2-box complex to 3.35A resolution. The structure reveals a remarkable four-way, protein-DNA bridged complex in which both ParB helix-turn-helix domains simultaneously bind adjacent A-boxes and the dimer domain bridges between two B-boxes. The multibridging capability and the novel dimer domain-B-box interaction, which juxtaposes the DNA sites close in space, suggests a mechanism for the formation of the wrapped solenoid-like segrosome superstructure. This multibridging capability of ParB is likely critical in its partition complex formation and pairing functions.