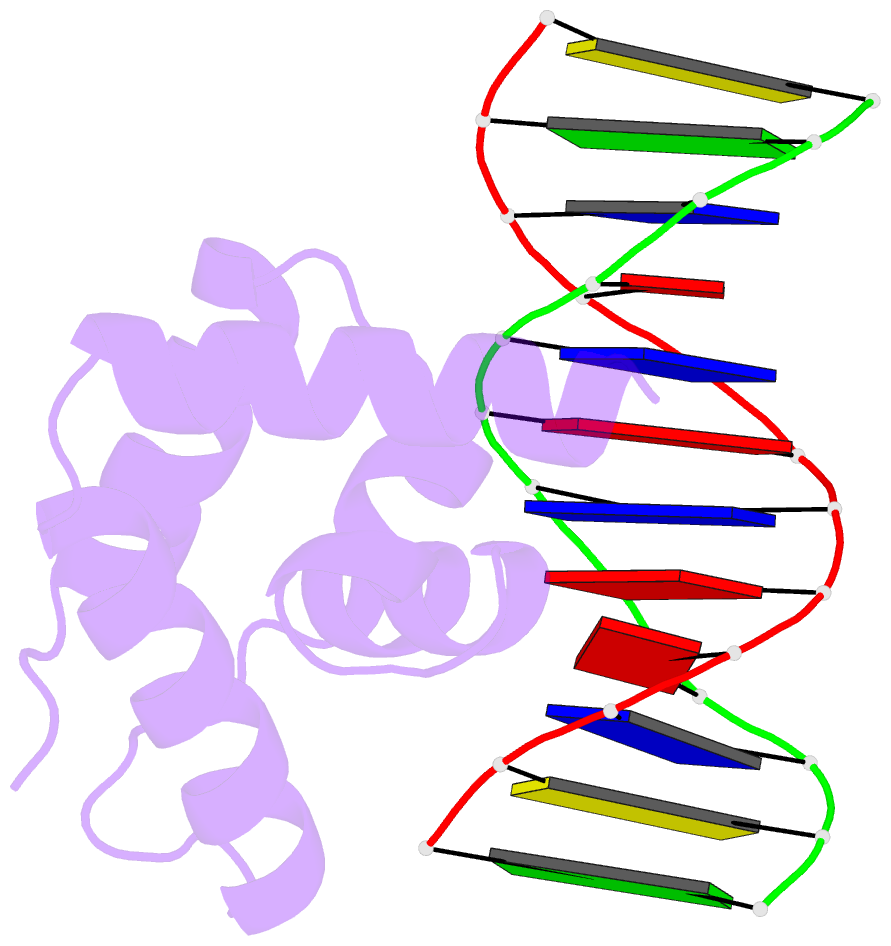
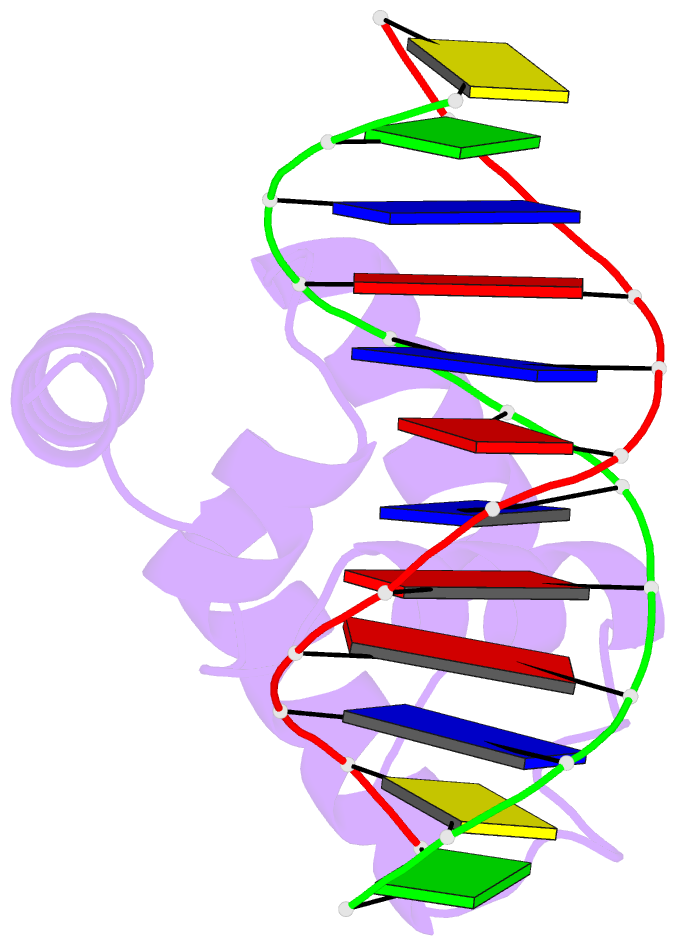
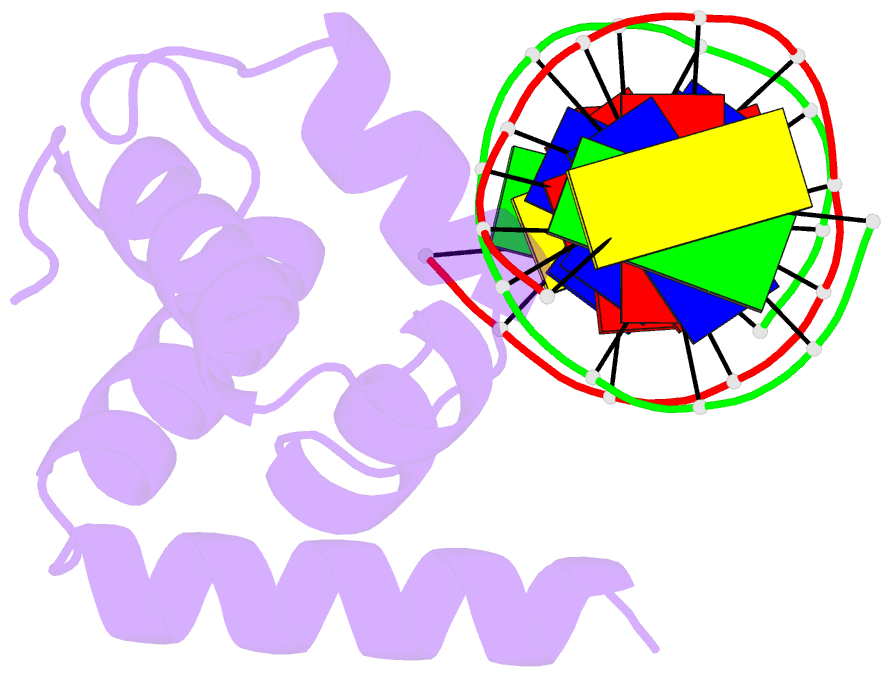
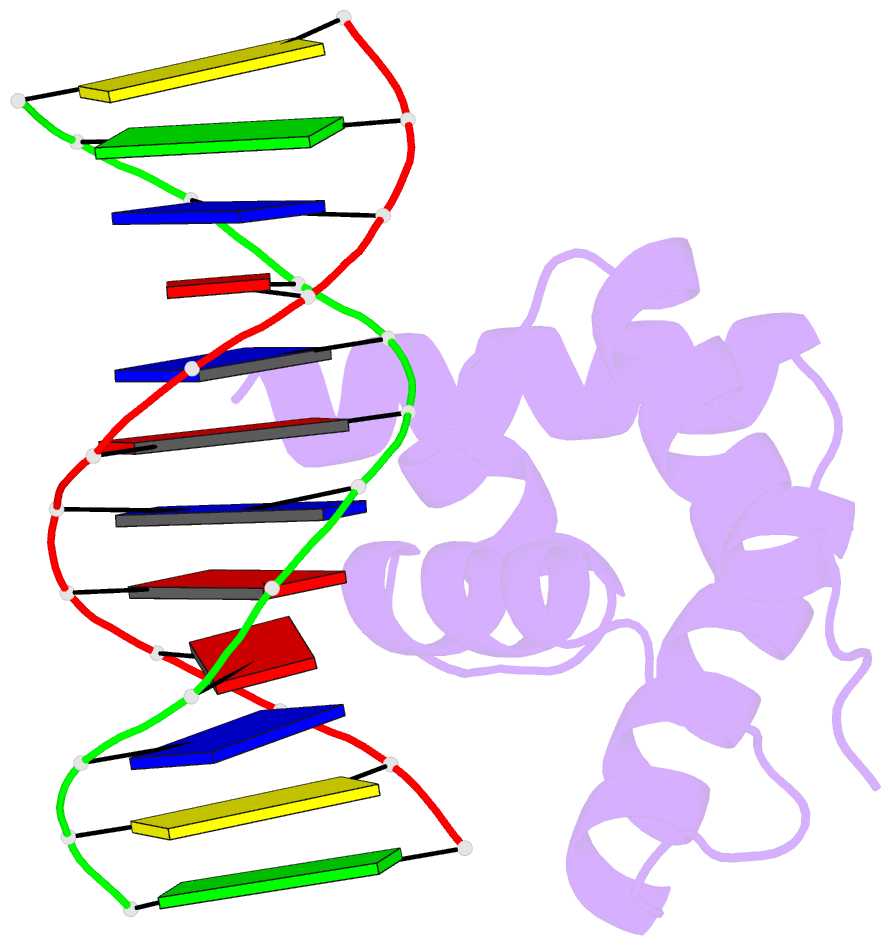
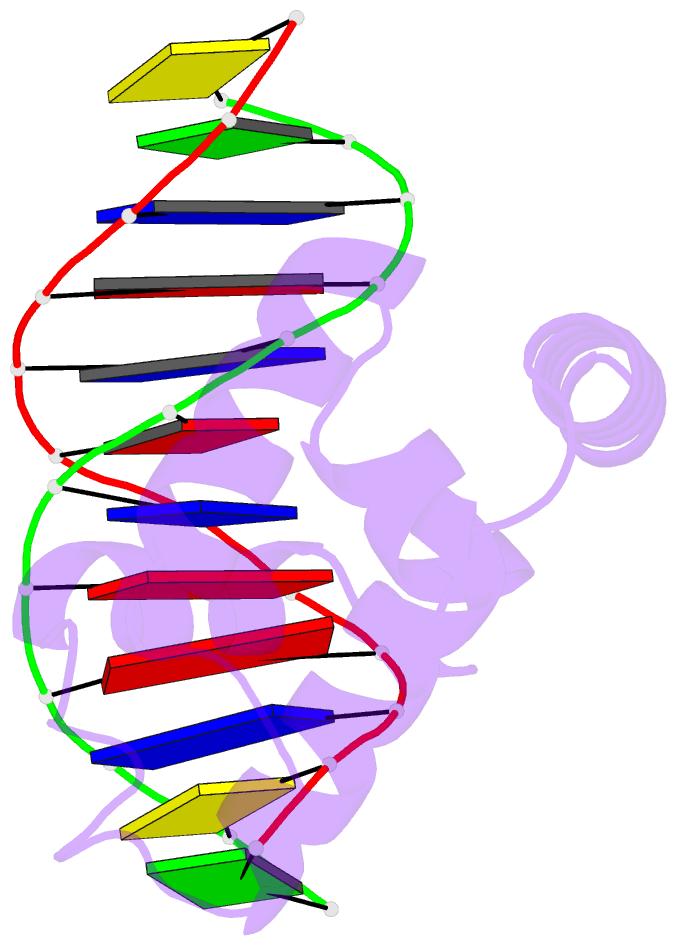
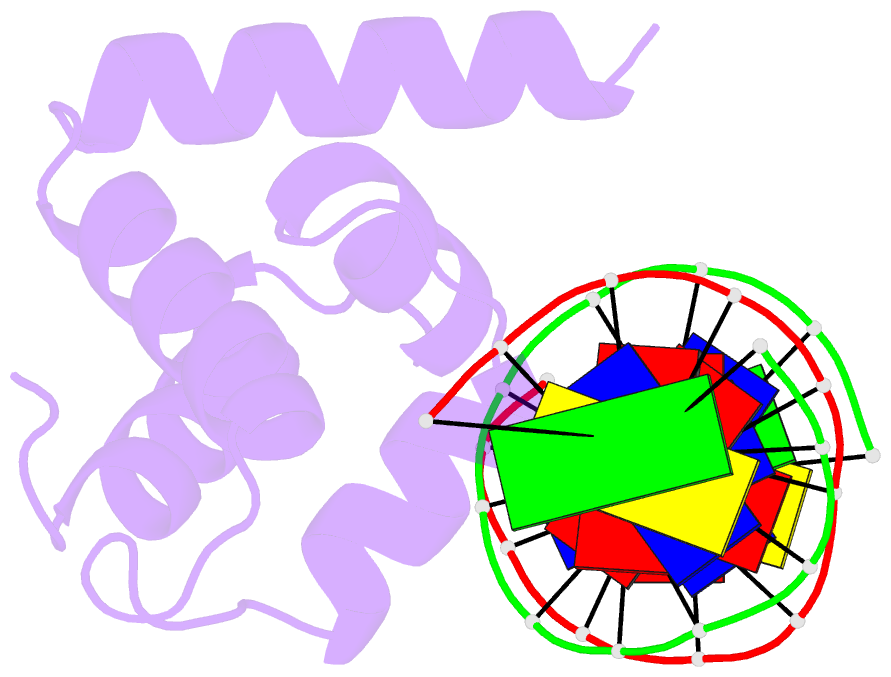
Summary information and primary citation
- PDB-id
- 2o4a; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (1.75 Å)
- Summary
- Crystal structure of the n-terminal cut domain of satb1 bound to matrix attachment region DNA
- Reference
- Yamasaki K, Akiba T, Yamasaki T, Harata K (2007): "Structural basis for recognition of the matrix attachment region of DNA by transcription factor SATB1." Nucleic Acids Res., 35, 5073-5084. doi: 10.1093/nar/gkm504.
- Abstract
- Special AT-rich sequence binding protein 1 (SATB1) regulates gene expression essential in immune T-cell maturation and switching of fetal globin species, by binding to matrix attachment regions (MARs) of DNA and inducing a local chromatin remodeling. Previously we have revealed a five-helix structure of the N-terminal CUT domain, which is essentially the folded region in the MAR-binding domain, of human SATB1 by NMR. Here we determined crystal structure of the complex of the CUT domain and a MAR DNA, in which the third helix of the CUT domain deeply enters the major groove of DNA in the B-form. Bases of 5'-CTAATA-3' sequence are contacted by this helix, through direct and water-mediated hydrogen bonds and apolar and van der Waals contacts. Mutations at conserved base-contacting residues, Gln402 and Gly403, reduced the DNA-binding activity, which confirmed the importance of the observed interactions involving these residues. A significant number of equivalent contacts are observed also for typically four-helix POU-specific domains of POU-homologous proteins, indicating that these domains share a common framework of the DNA-binding mode, recognizing partially similar DNA sequences.