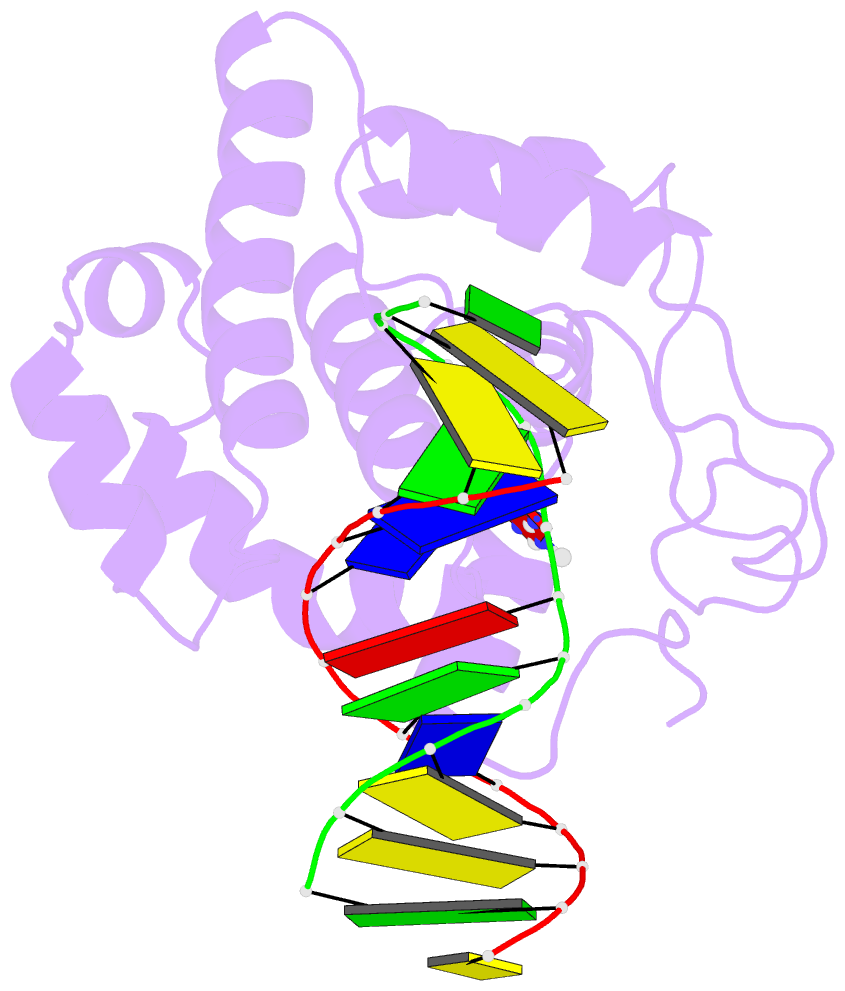
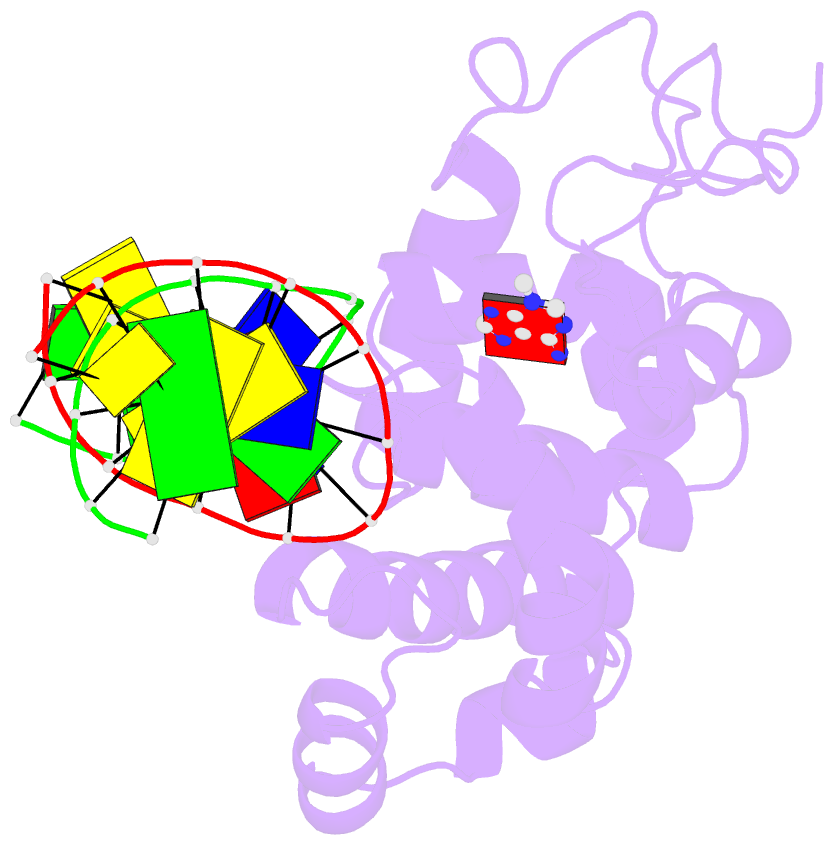
Summary information and primary citation
- PDB-id
- 2ofi; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- 3-methyladenine DNA glycosylase i-DNA
- Method
- X-ray (1.85 Å)
- Summary
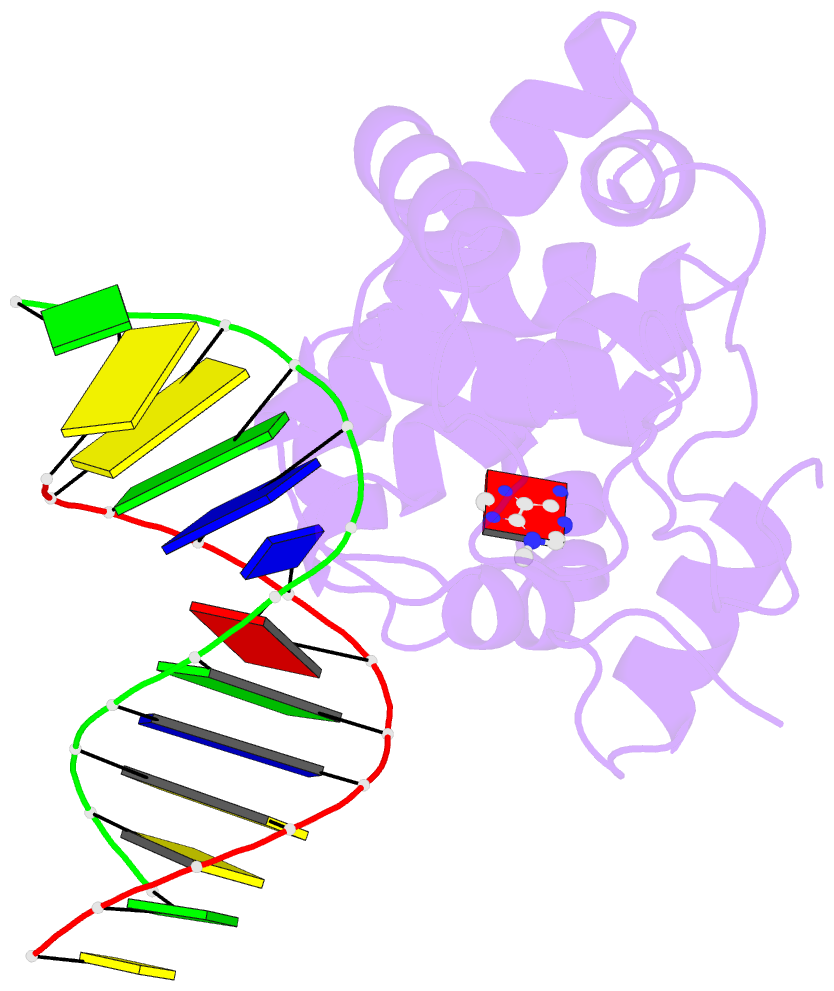
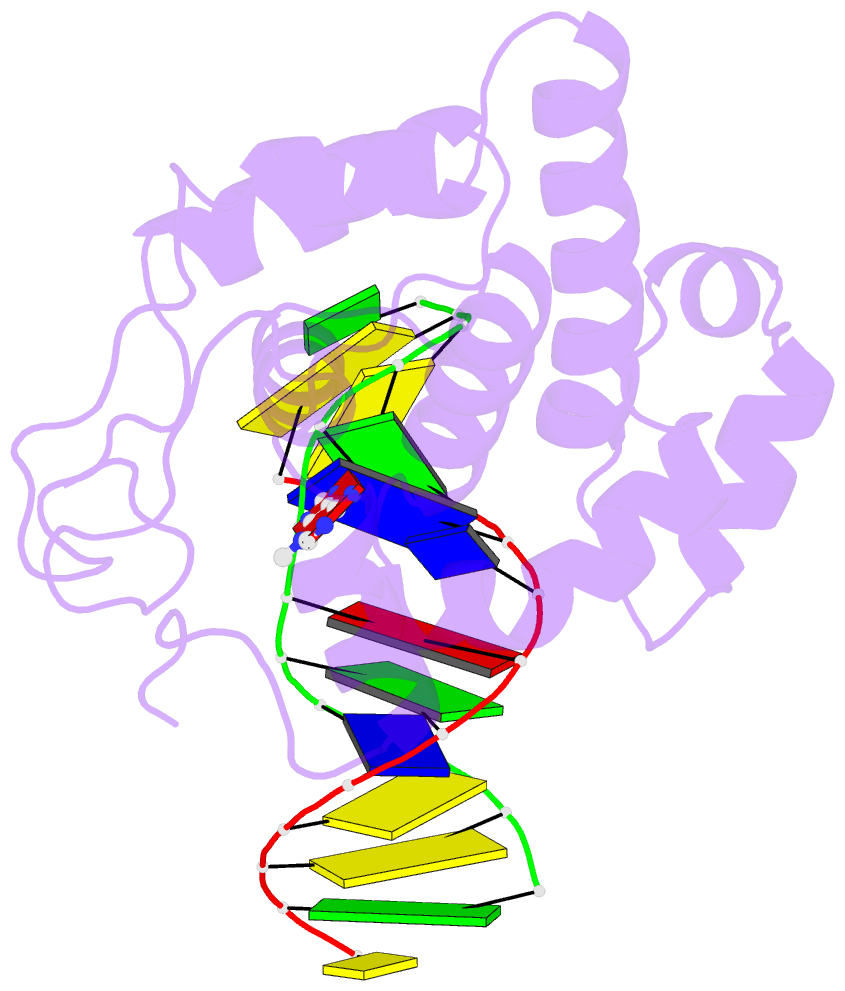
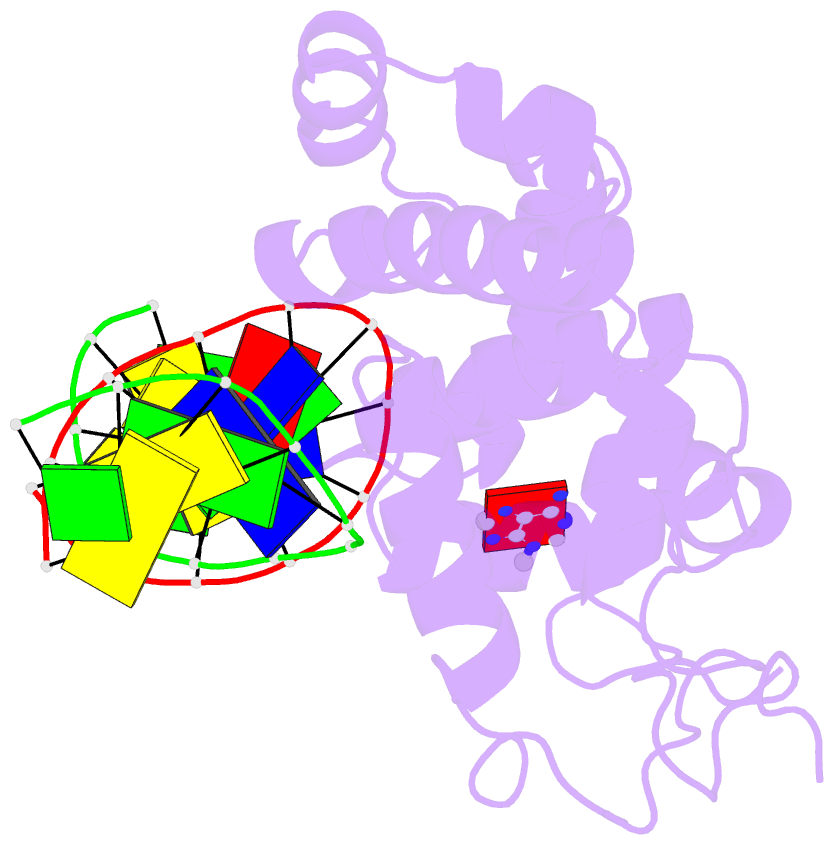
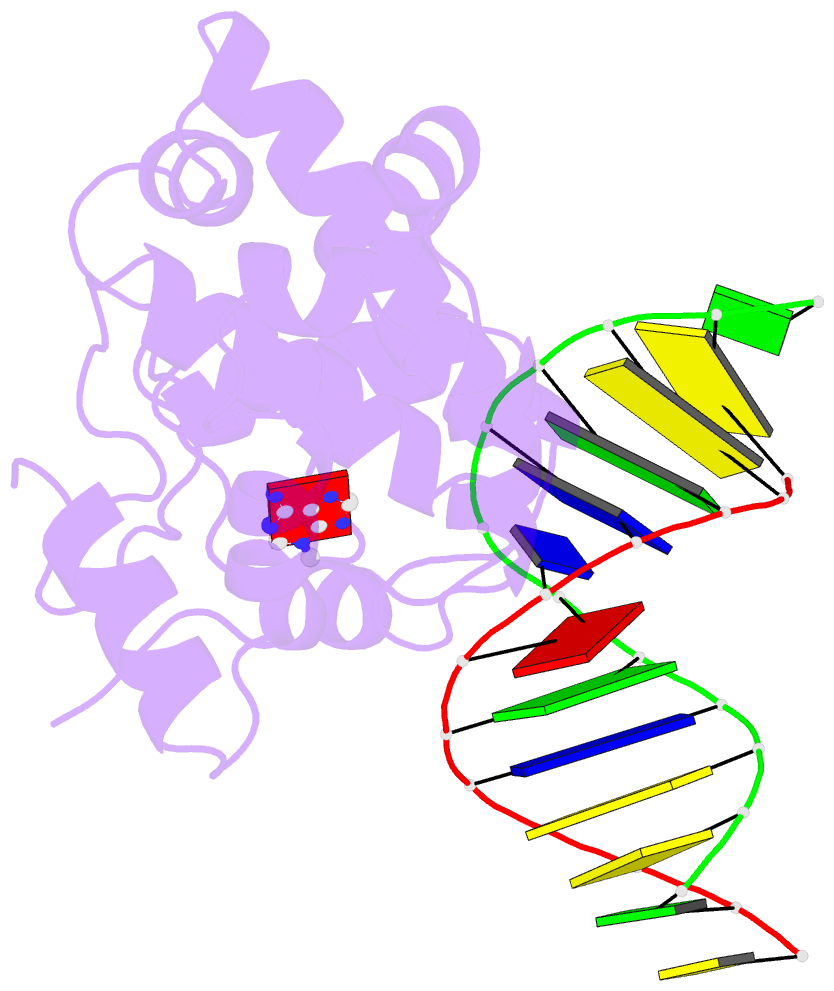
- Crystal structure of 3-methyladenine DNA glycosylase i (tag) bound to DNA-3ma
- Reference
- Metz AH, Hollis T, Eichman BF (2007): "DNA damage recognition and repair by 3-methyladenine DNA glycosylase I (TAG)." Embo J., 26, 2411-2420. doi: 10.1038/sj.emboj.7601649.
- Abstract
- DNA glycosylases help maintain the genome by excising chemically modified bases from DNA. Escherichia coli 3-methyladenine DNA glycosylase I (TAG) specifically catalyzes the removal of the cytotoxic lesion 3-methyladenine (3mA). The molecular basis for the enzymatic recognition and removal of 3mA from DNA is currently a matter of speculation, in part owing to the lack of a structure of a 3mA-specific glycosylase bound to damaged DNA. Here, high-resolution crystal structures of Salmonella typhi TAG in the unliganded form and in a ternary product complex with abasic DNA and 3mA nucleobase are presented. Despite its structural similarity to the helix-hairpin-helix superfamily of DNA glycosylases, TAG has evolved a modified strategy for engaging damaged DNA. In contrast to other glycosylase-DNA structures, the abasic ribose is not flipped into the TAG active site. This is the first structural demonstration that conformational relaxation must occur in the DNA upon base hydrolysis. Together with mutational studies of TAG enzymatic activity, these data provide a model for the specific recognition and hydrolysis of 3mA from DNA.