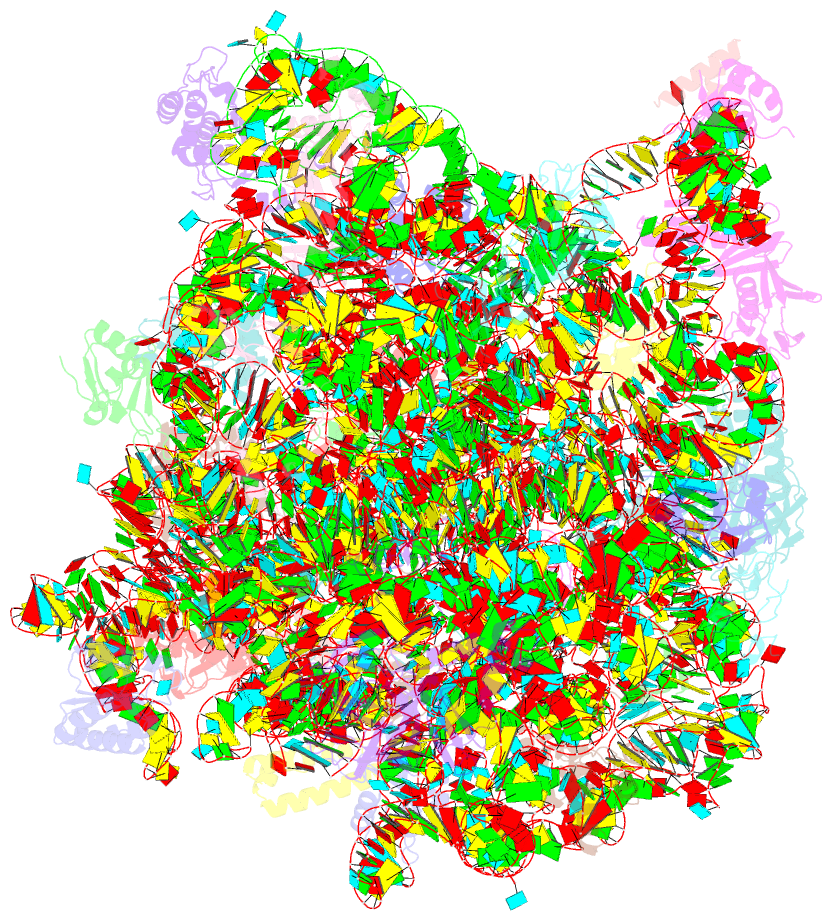
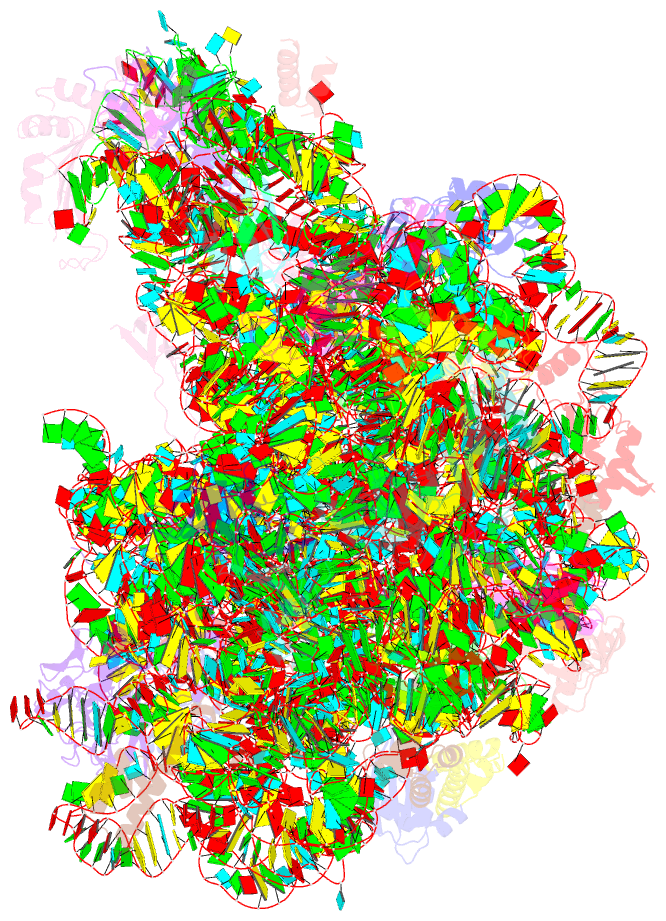
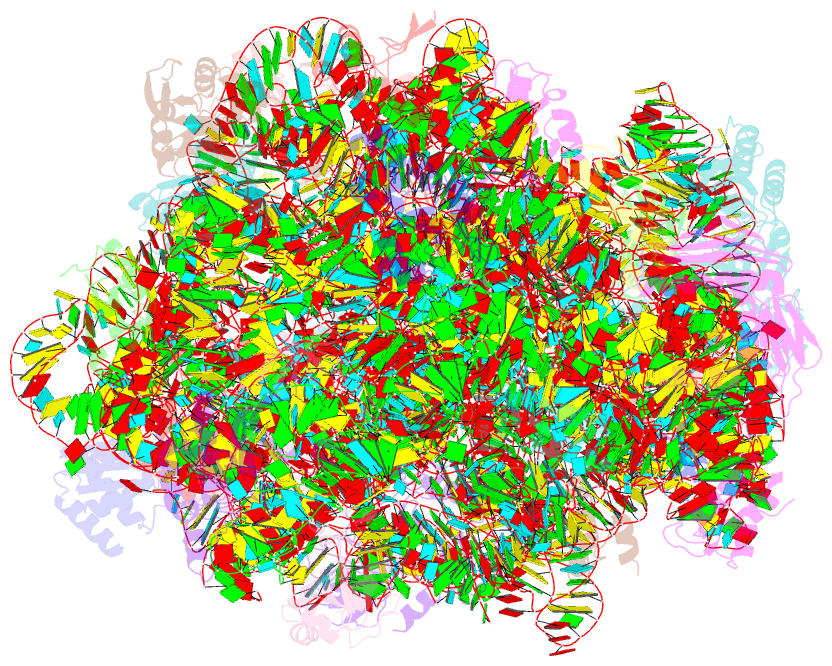
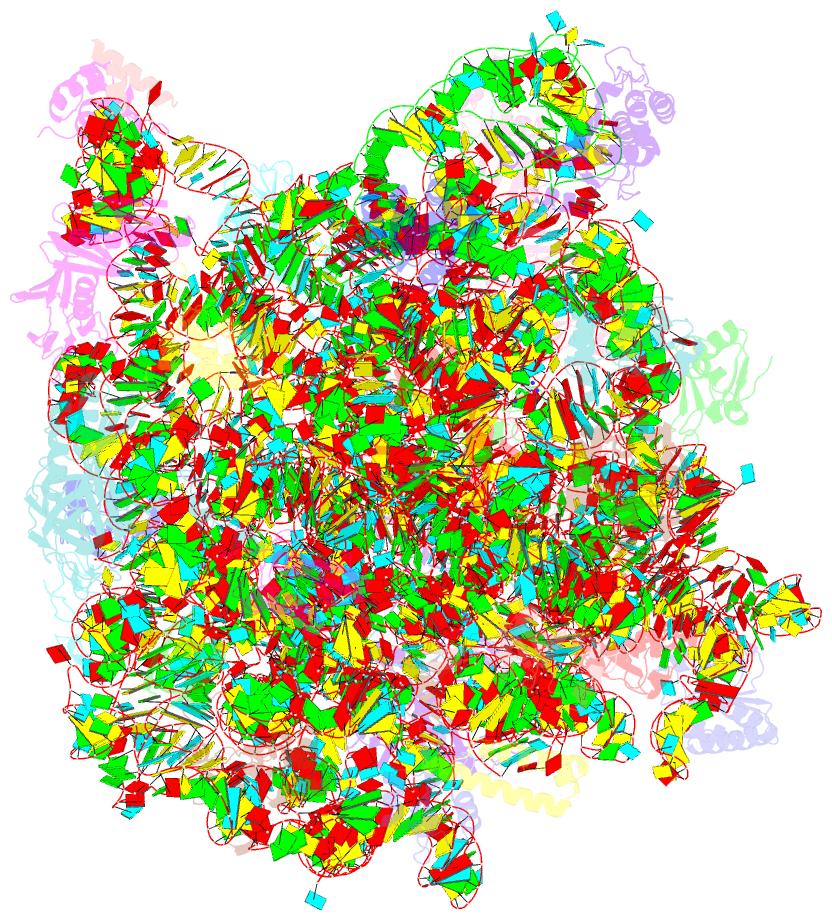
Summary information and primary citation
- PDB-id
- 2otl; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (2.7 Å)
- Summary
- Girodazole bound to the large subunit of haloarcula marismortui
- Reference
- Schroeder SJ, Blaha G, Tirado-Rives J, Steitz TA, Moore PB (2007): "The Structures of Antibiotics Bound to the E Site Region of the 50 S Ribosomal Subunit of Haloarcula marismortui: 13-Deoxytedanolide and Girodazole." J.Mol.Biol., 367, 1471-1479. doi: 10.1016/j.jmb.2007.01.081.
- Abstract
- Crystal structures of the 50 S ribosomal subunit from Haloarcula marismortui complexed with two antibiotics have identified new sites at which antibiotics interact with the ribosome and inhibit protein synthesis. 13-Deoxytedanolide binds to the E site of the 50 S subunit at the same location as the CCA of tRNA, and thus appears to inhibit protein synthesis by competing with deacylated tRNAs for E site binding. Girodazole binds near the E site region, but is somewhat buried and may inhibit tRNA binding by interfering with conformational changes that occur at the E site. The specificity of 13-deoxytedanolide for eukaryotic ribosomes is explained by its extensive interactions with protein L44e, which is an E site component of archaeal and eukaryotic ribosomes, but not of eubacterial ribosomes. In addition, protein L28, which is unique to the eubacterial E site, overlaps the site occupied by 13-deoxytedanolide, precluding its binding to eubacterial ribosomes. Girodazole is specific for eukarytes and archaea because it makes interactions with L15 that are not possible in eubacteria.