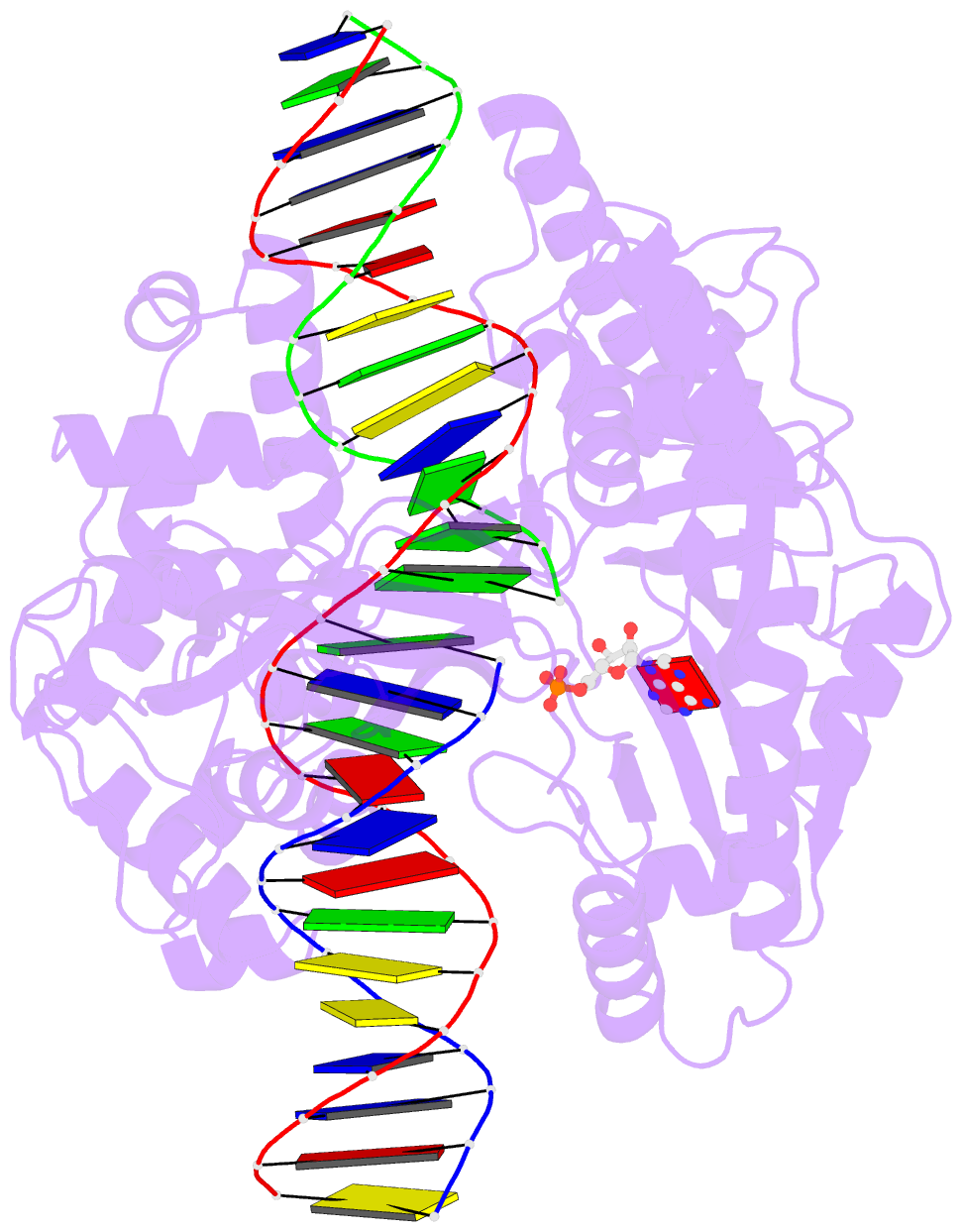
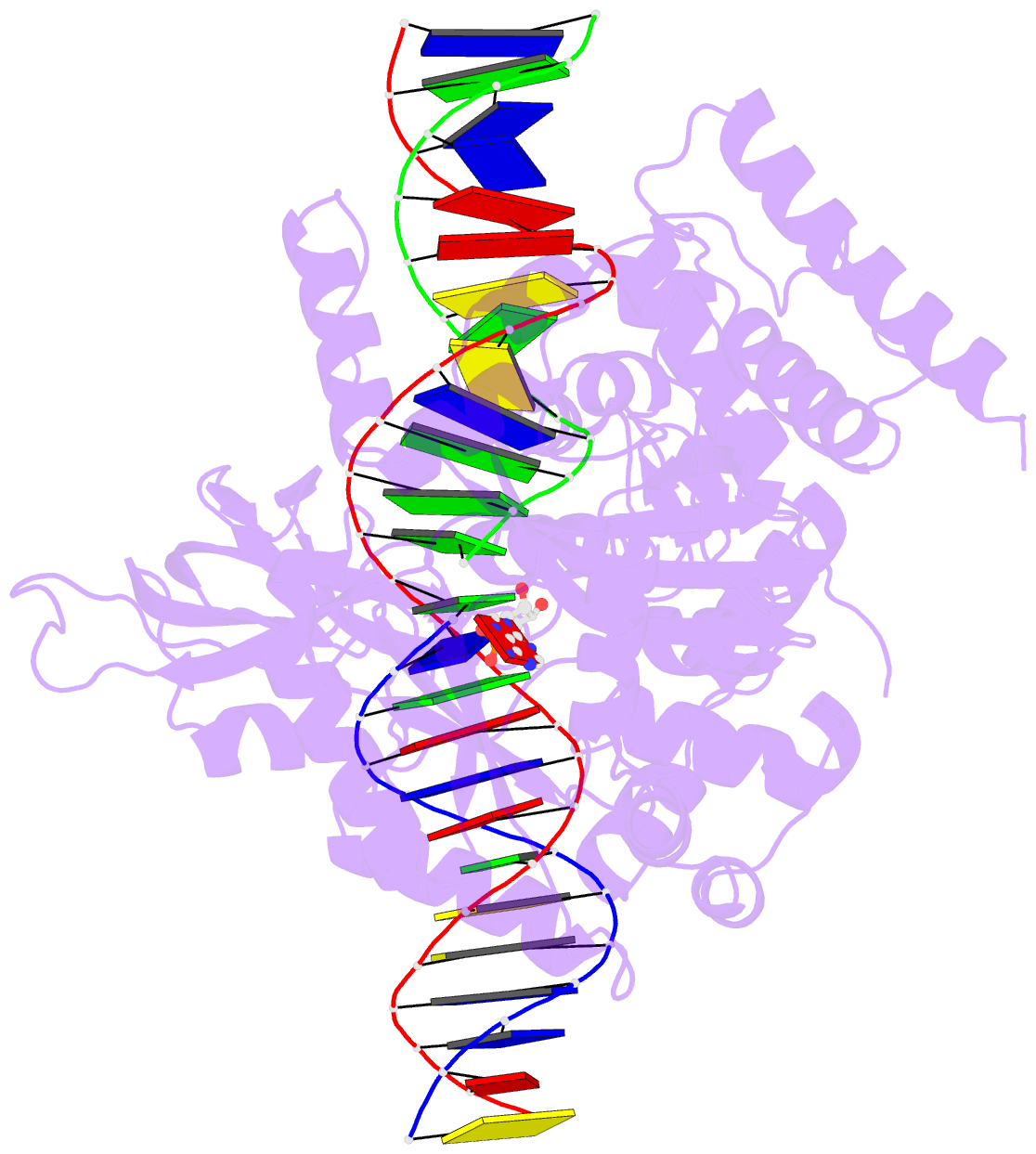
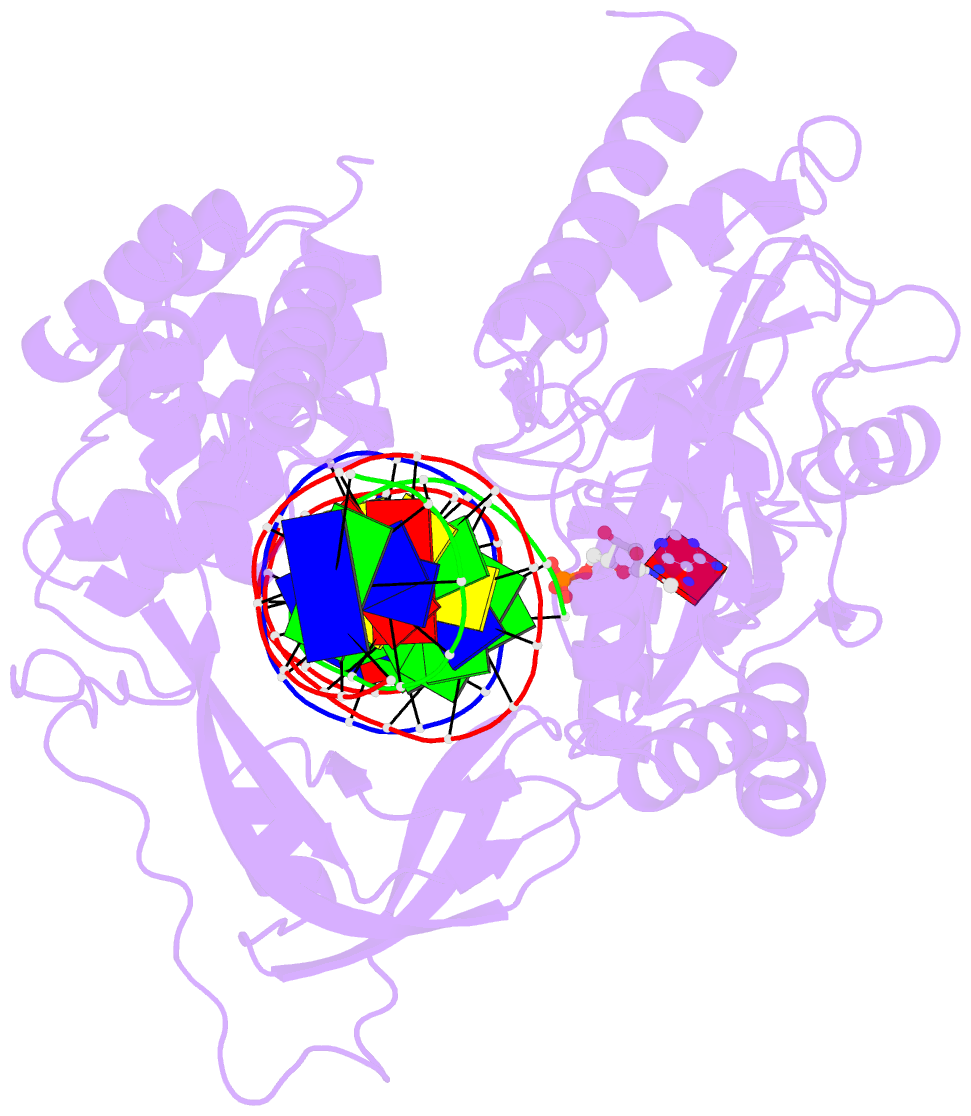
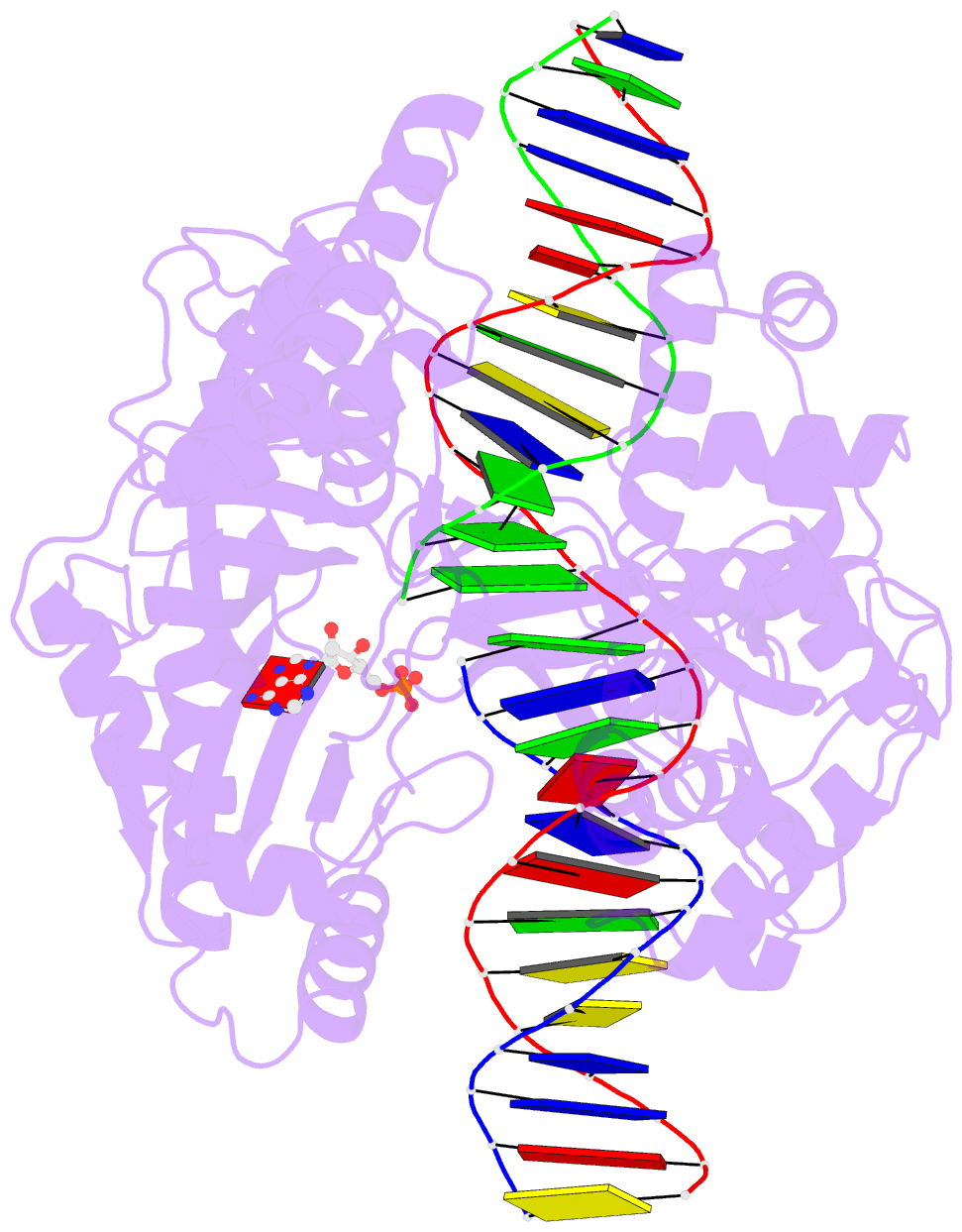
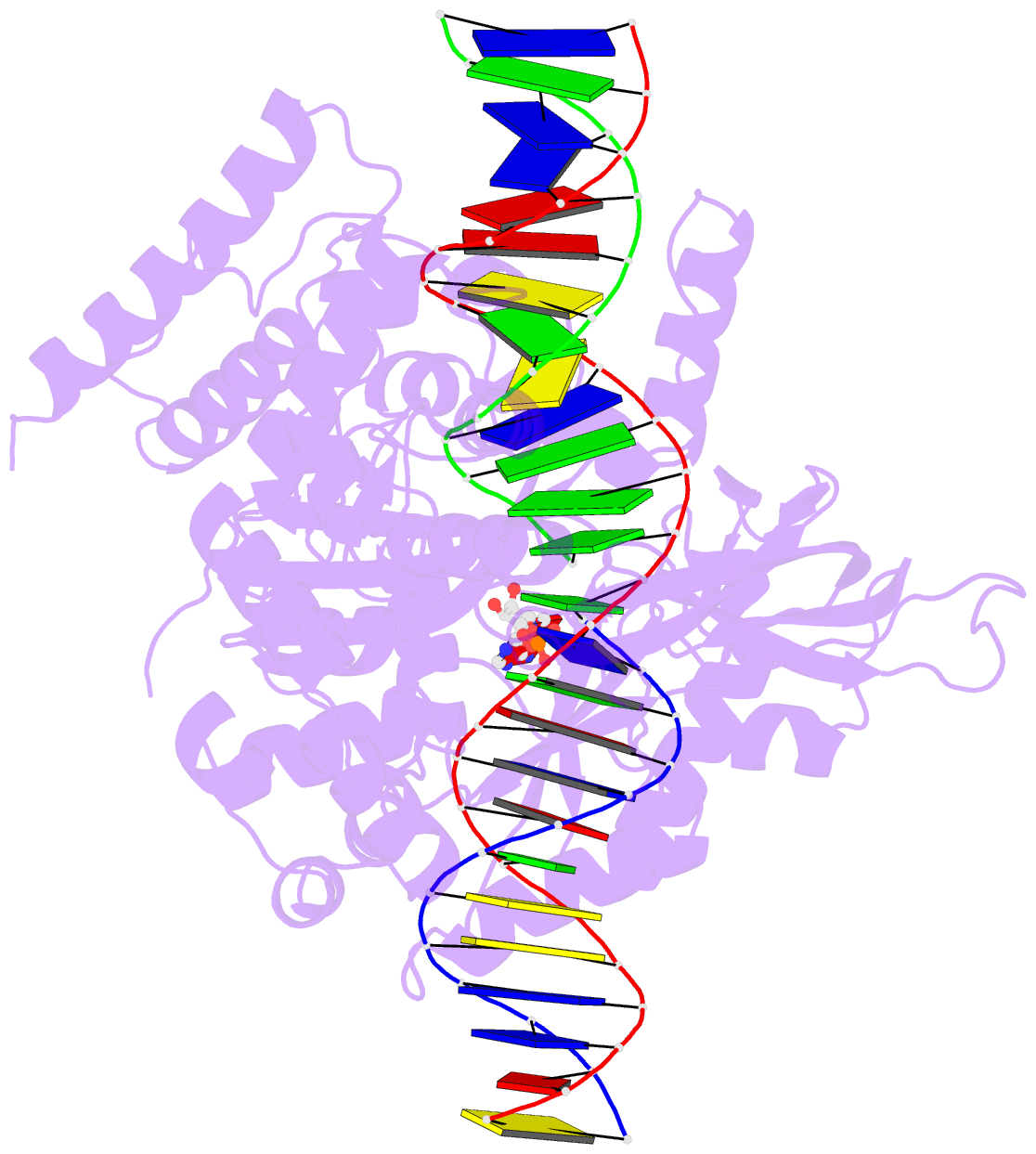
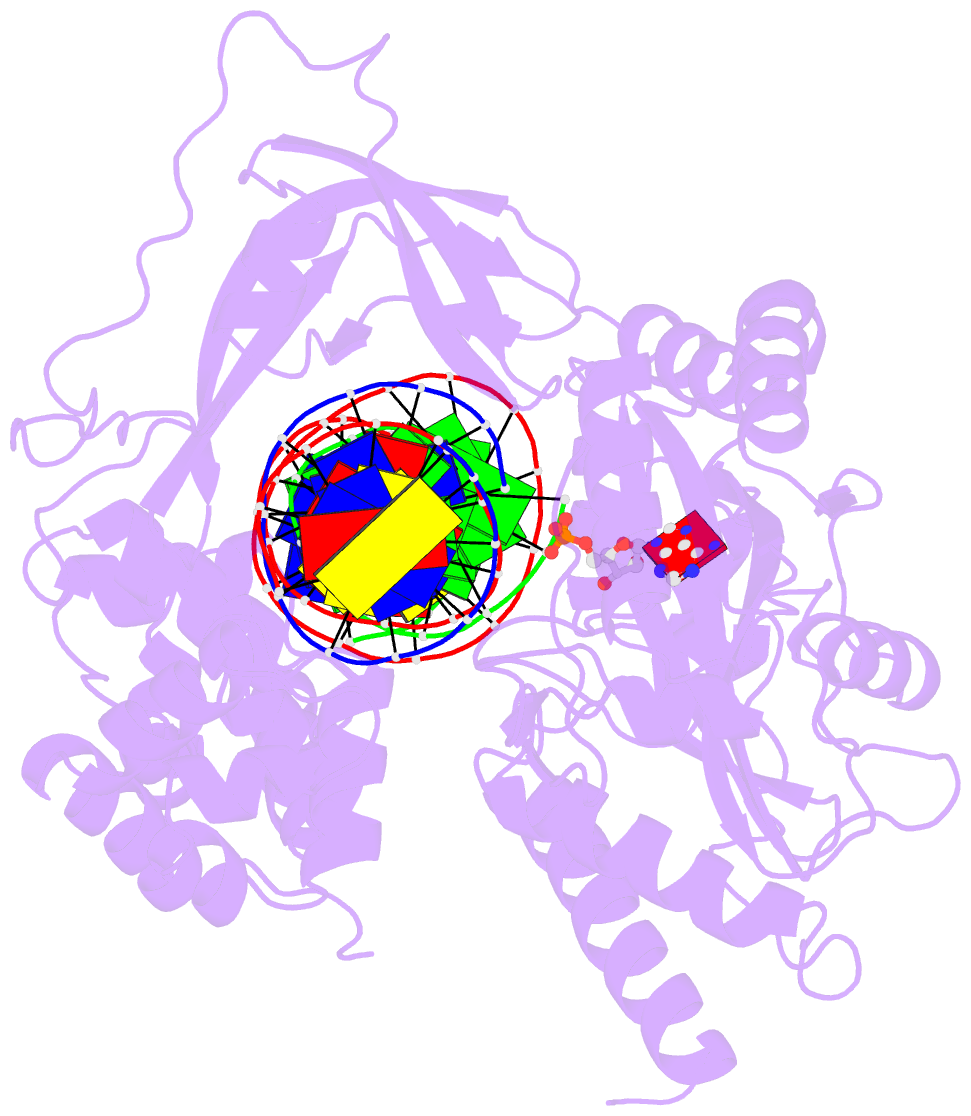
Summary information and primary citation
- PDB-id
- 2owo; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-DNA
- Method
- X-ray (2.3 Å)
- Summary
- Last stop on the road to repair: structure of e.coli DNA ligase bound to nicked DNA-adenylate
- Reference
- Nandakumar J, Nair PA, Shuman S (2007): "Last Stop on the Road to Repair: Structure of E. coli DNA Ligase Bound to Nicked DNA-Adenylate." Mol.Cell, 26, 257-271. doi: 10.1016/j.molcel.2007.02.026.
- Abstract
- NAD(+)-dependent DNA ligases (LigA) are ubiquitous in bacteria and essential for growth. Their distinctive substrate specificity and domain organization vis-a-vis human ATP-dependent ligases make them outstanding targets for anti-infective drug discovery. We report here the 2.3 A crystal structure of Escherichia coli LigA bound to an adenylylated nick, which captures LigA in a state poised for strand closure and reveals the basis for nick recognition. LigA envelopes the DNA within a protein clamp. Large protein domain movements and remodeling of the active site orchestrate progression through the three chemical steps of the ligation reaction. The structure inspires a strategy for inhibitor design.