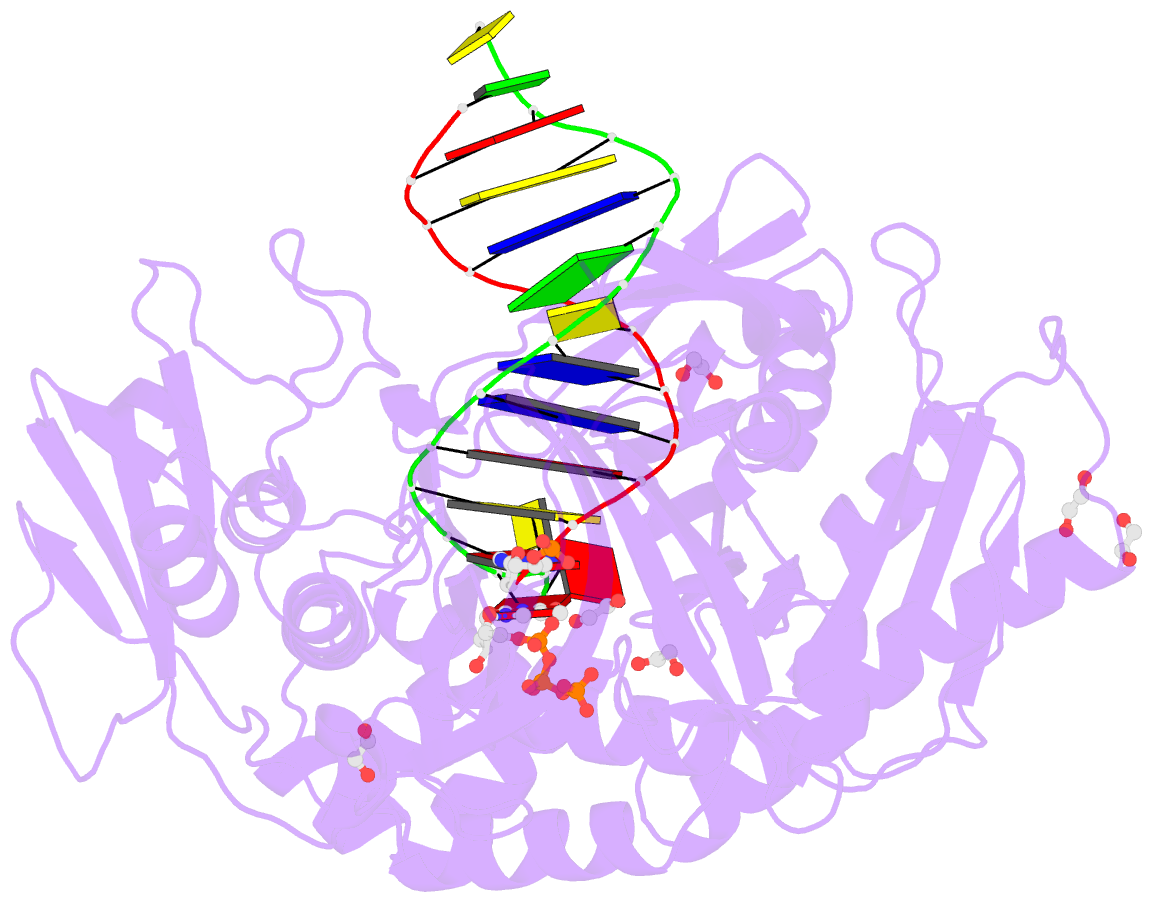
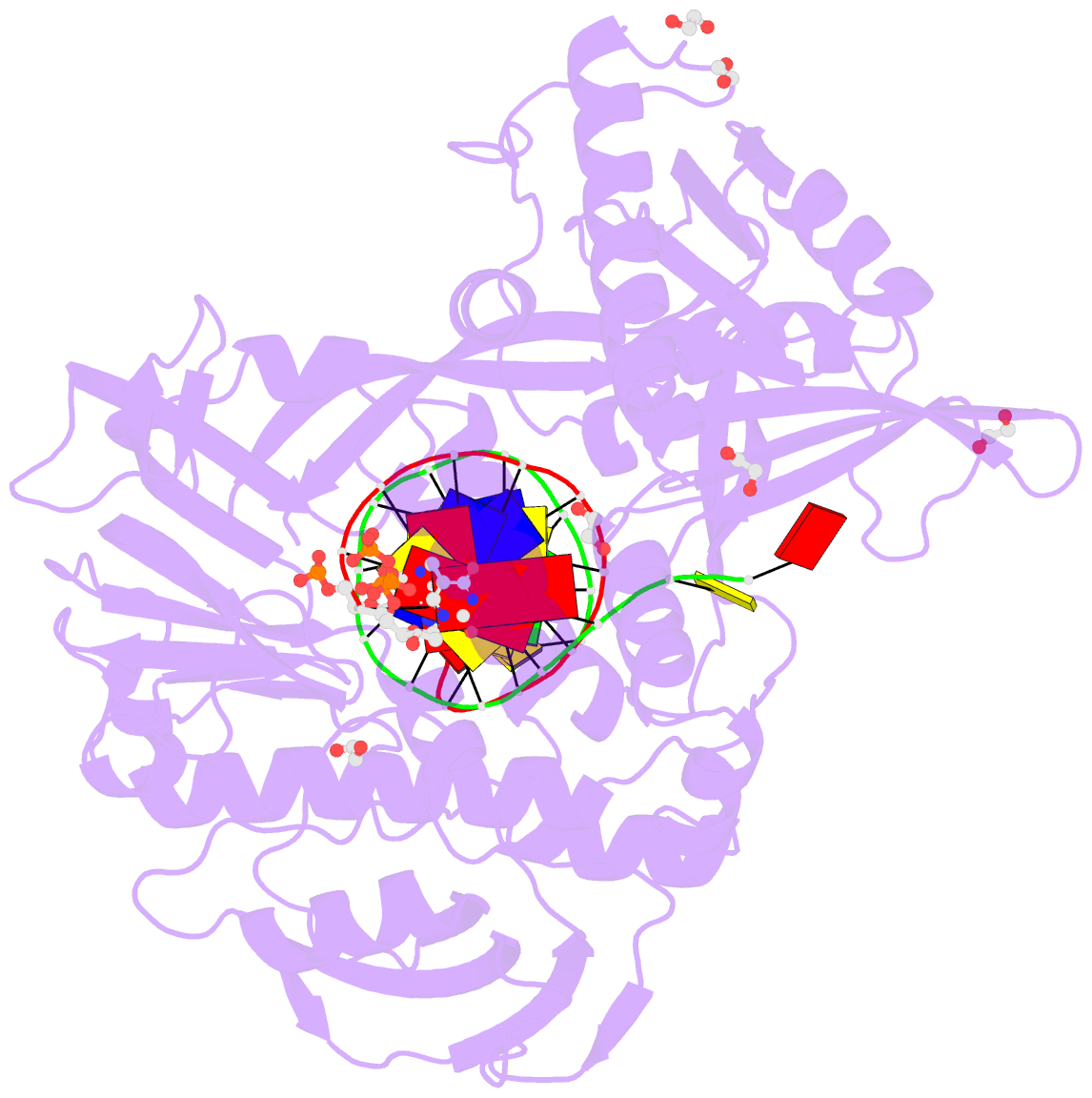
Summary information and primary citation
- PDB-id
- 2pyl; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication, transferase-DNA
- Method
- X-ray (2.2 Å)
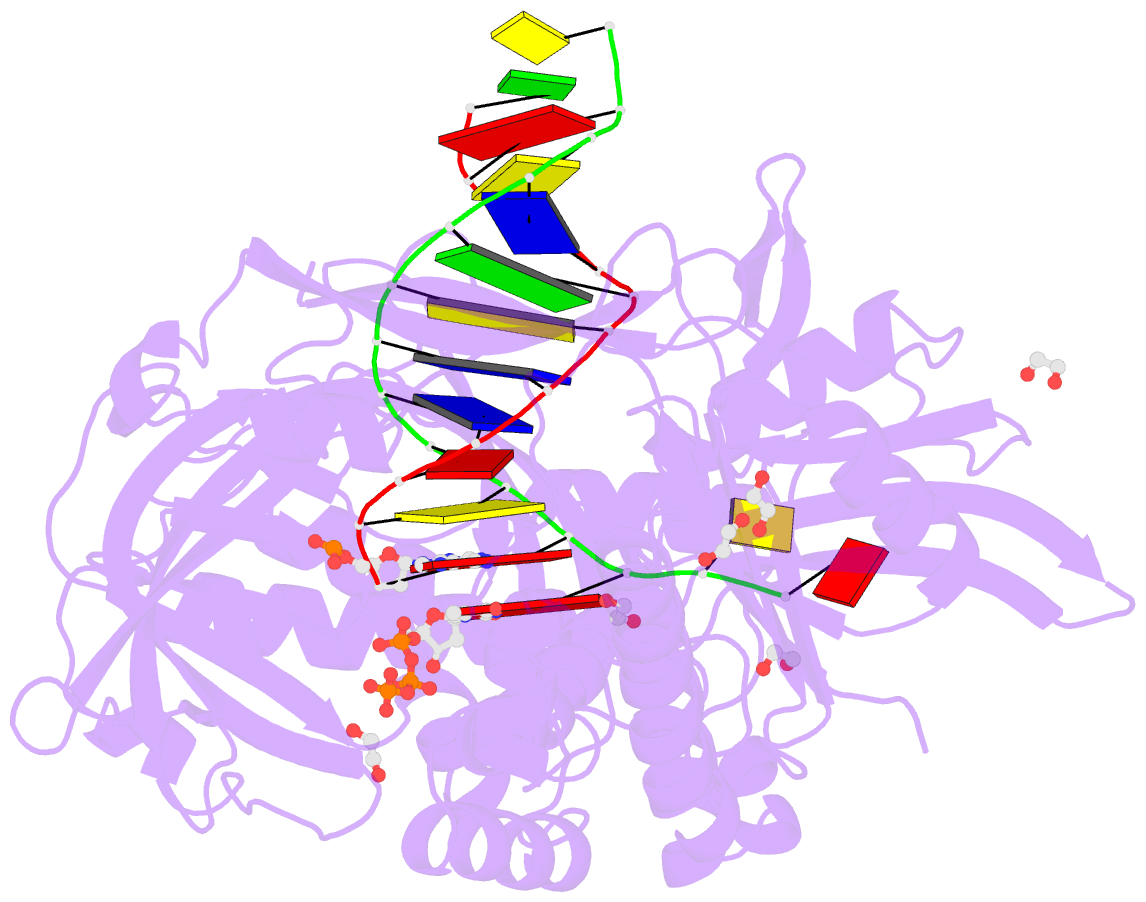
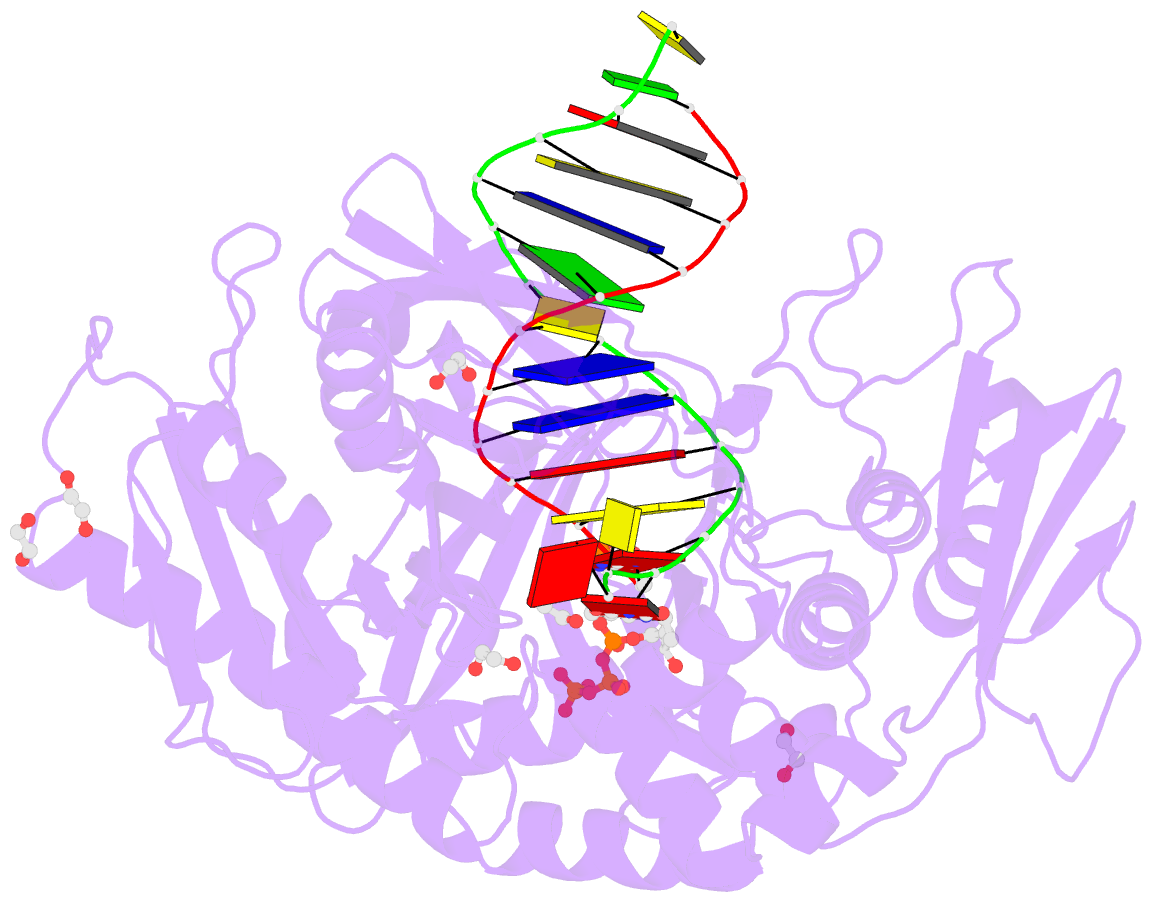
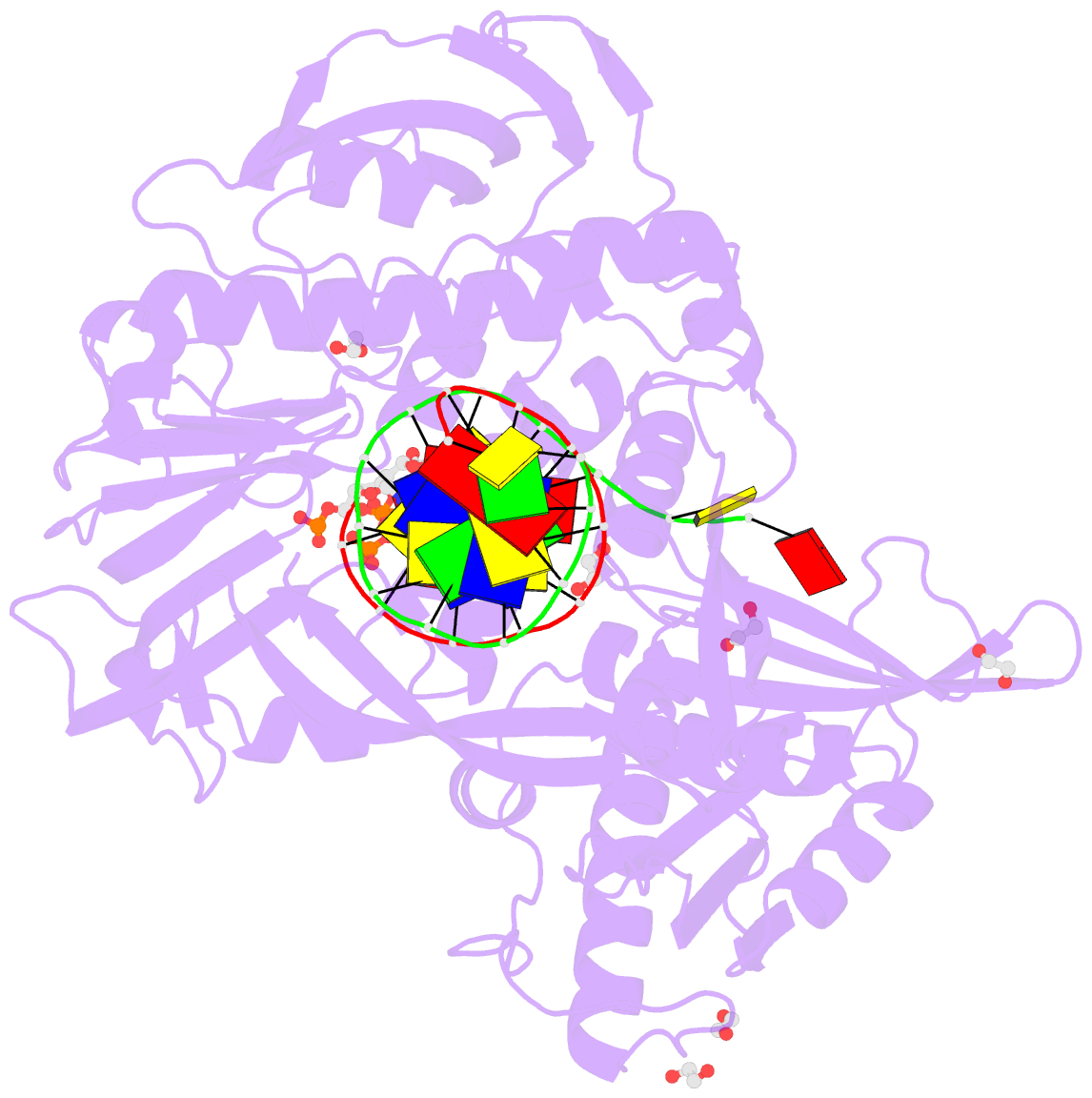
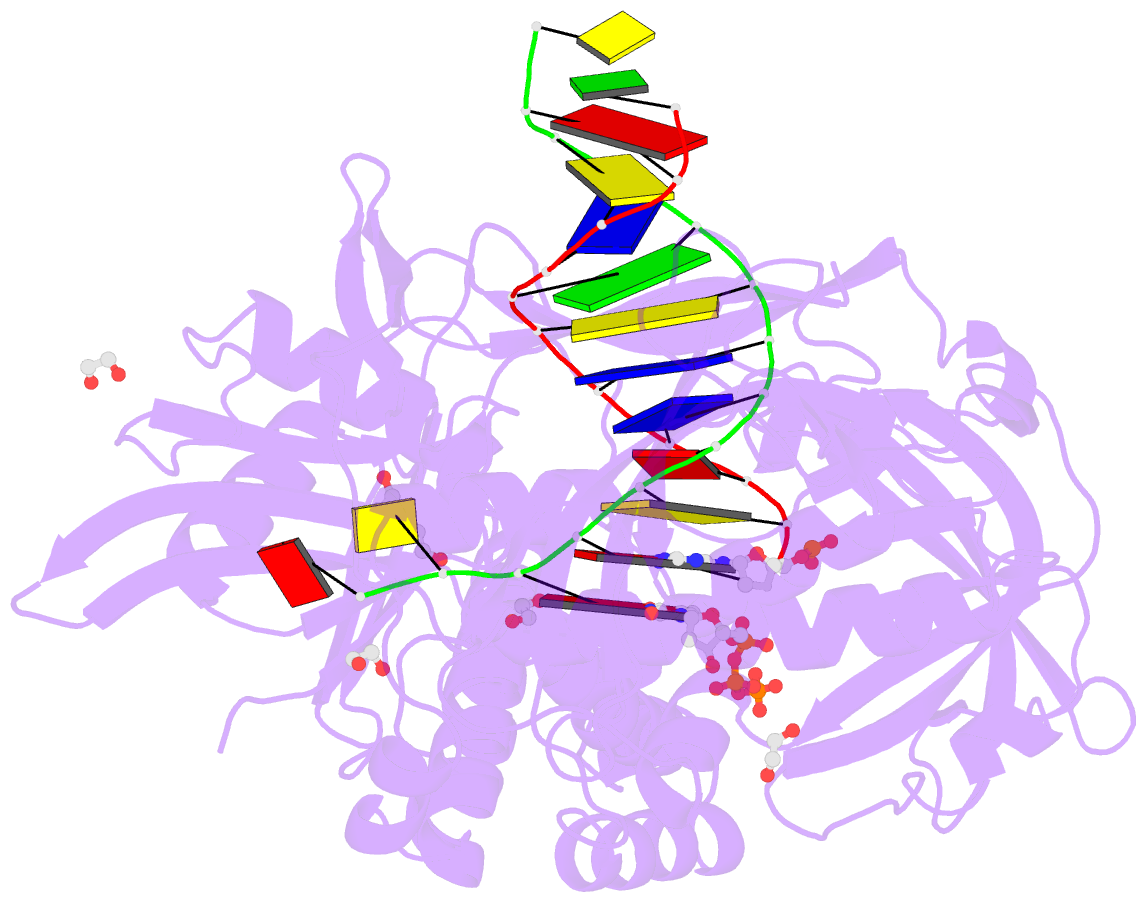
- Summary
- Phi29 DNA polymerase complexed with primer-template DNA and incoming nucleotide substrates (ternary complex)
- Reference
- Berman AJ, Kamtekar S, Goodman JL, Lazaro JM, de Vega M, Blanco L, Salas M, Steitz TA (2007): "Structures of phi29 DNA polymerase complexed with substrate: the mechanism of translocation in B-family polymerases." Embo J., 26, 3494-3505. doi: 10.1038/sj.emboj.7601780.
- Abstract
- Replicative DNA polymerases (DNAPs) move along template DNA in a processive manner. The structural basis of the mechanism of translocation has been better studied in the A-family of polymerases than in the B-family of replicative polymerases. To address this issue, we have determined the X-ray crystal structures of phi29 DNAP, a member of the protein-primed subgroup of the B-family of polymerases, complexed with primer-template DNA in the presence or absence of the incoming nucleoside triphosphate, the pre- and post-translocated states, respectively. Comparison of these structures reveals a mechanism of translocation that appears to be facilitated by the coordinated movement of two conserved tyrosine residues into the insertion site. This differs from the mechanism employed by the A-family polymerases, in which a conserved tyrosine moves into the templating and insertion sites during the translocation step. Polymerases from the two families also interact with downstream single-stranded template DNA in very different ways.